Abstract
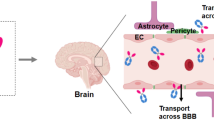
Biologics are potential new therapeutics for many diseases of the central nervous system. Biologics include recombinant lysosomal enzymes, neurotrophins, decoy receptors, and therapeutic antibodies. These are large molecule drugs that do not cross the blood–brain barrier (BBB). All classes of biologics have been tested, without success, in clinical trials of brain disease over the last 25 years. In none of these past clinical trials was the biologic re-engineered to enable transport across the BBB. If the biologic does not cross the BBB, the drug cannot reach the target site in brain, and success in a clinical trial is not expected. Biologics can be re-engineered for BBB transport with the use of molecular Trojan horse technology. A BBB molecular Trojan horse is a monoclonal antibody (MAb) against an endogenous BBB receptor transporter, such as the insulin receptor or transferrin receptor. The receptor-specific MAb penetrates the brain via transport on the endogenous BBB receptor. The MAb acts as a molecular Trojan horse to deliver across the BBB the biologic pharmaceutical that is genetically fused to the MAb. The lead Trojan horse is a MAb against the human insulin receptor (HIR), and HIRMAb-derived fusion proteins have entered clinical trials for the treatment of brain disease.




Similar content being viewed by others
References
Pardridge WM. Targeted delivery of protein and gene medicines through the blood–brain barrier. Clin Pharmacol Ther. 2015;97(4):347–61.
Cadavid D, Jurgensen S, Lee S. Impact of natalizumab on ambulatory improvement in secondary progressive and disabled relapsing-remitting multiple sclerosis. PLoS One. 2013;8(1):e53297.
Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8(1):4.
Han K, Ren M, Wick W, Abrey L, Das A, Jin J, et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5):696–706.
Liu HL, Hsu PH, Lin CY, Huang CW, Chai WY, Chu PC, et al. Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology. 2016;281(1):99–108.
Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood–brain barrier. Methods Enzymol. 2012;503:269–92.
Sakane T, Pardridge WM. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm Res. 1997;14(8):1085–91.
Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH 2nd, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18(3):179–90.
Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood–brain barrier and blood–spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;09(1157):126–37.
The BDNF Study Group (Phase III). A controlled trial of recombinant methionyl human BDNF in ALS. Neurology. 1999;52(7):1427–33.
Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, Goodpasture JC, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann Neurol. 1996;39(2):256–60.
Lyons MK, Anderson RE, Meyer FB. Basic fibroblast growth factor promotes in vivo cerebral angiogenesis in chronic forebrain ischemia. Brain Res. 1991;558(2):315–20.
Fisher M, Meadows ME, Do T, Weise J, Trubetskoy V, Charette M, Finklestein SP. Delayed treatment with intravenous basic fibroblast growth factor reduces infarct size following permanent focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1995;15:953–9.
Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood–brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56(4):468–77.
Bogousslavsky J, Victor SJ, Salinas EO, Pallay A, Donnan GA, Fieschi C, et al. Fiblast (trafermin) in acute stroke: results of the European-Australian phase II/III safety and efficacy trial. Cerebrovasc Dis. 2002;14(3–4):239–51.
Zivin JA. Factors determining the therapeutic window for stroke. Neurology. 1998;50(3):599–603.
Schlageter NL, Carson RE, Rapoport SI. Examination of blood–brain barrier permeability in dementia of the Alzheimer type with [68 Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7(1):1–8.
Azim HA, Azim HA Jr. Systemic treatment of brain metastases in HER2-positive breast cancer: current status and future directions. Future Oncol. 2012;8(2):135–44.
Pardridge WM. CSF, blood–brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–75.
Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3–4):79–96.
Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–56.
Boado RJ, Hui EK, Lu JZ, Pardridge WM. Drug targeting of erythropoietin across the primate blood–brain barrier with an IgG molecular Trojan horse. J Pharmacol Exp Ther. 2010;333(3):961–9.
Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97(19):10526–31.
Xenocostas A, Cheung WK, Farrell F, Zakszewski C, Kelley M, Lutynski A, et al. The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol. 2005;61(3):189–95.
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–2.
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER Jr, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73.
Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp Neurol. 1994;127(1):23–36.
Nagaraja TN, Patel P, Gorski M, Gorevic PD, Patlak CS, Fenstermacher JD. In normal rat, intraventricularly administered insulin-like growth factor-1 is rapidly cleared from CSF with limited distribution into brain. Cerebrospinal Fluid Res. 2005;26(2):5.
Kimelberg HK, Kung D, Watson RE, Reiss FL, Biddlecome SM, Bourke RS. Direct administration of methotrexate into the central nervous system of primates. Part 1: distribution and degradation of methotrexate in nervous and systemic tissue after intraventricular injection. J Neurosurg. 1978;48(6):883–94.
Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther. 1975;195(1):73–83.
Fishman RA, Christy NP. Fate of adrenal cortical steroids following intrathecal injection. Neurology. 1965;15:1–6.
Paton DM. Nusinersen: antisense oligonucleotide to increase SMN protein production in spinal muscular atrophy. Drugs Today (Barc). 2017;53(6):327–37.
Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, et al. Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther. 2014;350(1):46–55.
Vuillemenot BR, Kennedy D, Cooper JD, Wong AM, Sri S, Doeleman T, et al. Nonclinical evaluation of CNS-administered TPP1 enzyme replacement in canine CLN2 neuronal ceroid lipofuscinosis. Mol Genet Metab. 2015;114(2):281–93.
Romano PS, Carvelli L, Lopez AC, Jofre G, Sartor T, Sosa MA. Developmental differences between cation-independent and cation-dependent mannose-6-phosphate receptors in rat brain at perinatal stages. Brain Res Dev Brain Res. 2005;158(1–2):23–30.
Kohlschutter A, Schulz A. CLN2 disease (classic late infantile neuronal ceroid lipofuscinosis). Pediatr Endocrinol Rev. 2016;13(Suppl 1):682–8.
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59(3):459–66.
Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202(2):497–505.
Greven CU, Bralten J, Mennes M, O’Dwyer L, van Hulzen KJE, Rommelse N, et al. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatr. 2015;72(5):490–9.
Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet. 1995;346(8989):1524–8.
Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56(4):321–39.
Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci USA. 1997;94(8):4109–12.
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–9.
Seubert P, Barbour R, Khan K, Motter R, Tang P, Kholodenko D, et al. Antibody capture of soluble Abeta does not reduce cortical Abeta amyloidosis in the PDAPP mouse. Neurodegener Dis. 2008;5(2):65–71.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–33.
Zago W, Schroeter S, Guido T, Khan K, Seubert P, Yednock T, et al. Vascular alterations in PDAPP mice after anti-Abeta immunotherapy: implications for amyloid-related imaging abnormalities. Alzheimers Dement. 2013;9(5 Suppl):S105–15.
Barkhof F, Daams M, Scheltens P, Brashear HR, Arrighi HM, Bechten A, et al. An MRI rating scale for amyloid-related imaging abnormalities with edema or effusion. AJNR Am J Neuroradiol. 2013;34(8):1550–5.
Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241–9.
Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–6.
Sumbria RK, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Disaggregation of amyloid plaque in brain of Alzheimer’s disease transgenic mice with daily subcutaneous administration of a tetravalent bispecific antibody that targets the transferrin receptor and the Abeta amyloid peptide. Mol Pharm. 2013;10(9):3507–13.
Pardridge WM. Blood–brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19(8):1059–72.
Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood–brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem. 1990;265(29):18035–40.
Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273(1 Pt 1):E207–13.
Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood–brain barrier. Proc Natl Acad Sci USA. 1999;96(21):12079–84.
Stoll J, Wadhwani KC, Smith QR. Identification of the cationic amino acid transporter (System y+) of the rat blood–brain barrier. J Neurochem. 1993;60(5):1956–9.
Li JY, Boado RJ, Pardridge WM. Cloned blood–brain barrier adenosine transporter is identical to the rat concentrative Na+ nucleoside cotransporter CNT2. J Cereb Blood Flow Metab. 2001;21(8):929–36.
Cornford EM, Oldendorf WH. Independent blood–brain barrier transport systems for nucleic acid precursors. Biochim Biophys Acta. 1975;394(2):211–9.
Cornford EM, Braun LD, Oldendorf WH. Carrier mediated blood–brain barrier transport of choline and certain choline analogs. J Neurochem. 1978;30(2):299–308.
Zuchero YJ, Chen X, Bien-Ly N, Bumbaca D, Tong RK, Gao X, et al. Discovery of novel blood–brain barrier targets to enhance brain uptake of therapeutic antibodies. Neuron. 2016;89(1):70–82.
Frank HJ, Jankovic-Vokes T, Pardridge WM, Morris WL. Enhanced insulin binding to blood–brain barrier in vivo and to brain microvessels in vitro in newborn rabbits. Diabetes. 1985;34(8):728–33.
Giddings SJ, Chirgwin J, Permutt MA. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia. 1985;28(6):343–7.
Duffy KR, Pardridge WM. Blood–brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420(1):32–8.
Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [125I]transferrin through the rat blood–brain barrier. Brain Res. 1995;683(2):164–71.
Li JY, Boado RJ, Pardridge WM. Blood–brain barrier genomics. J Cereb Blood Flow Metab. 2001;21(1):61–8.
Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J Neurochem. 2001;76(5):1597–600.
Duffy KR, Pardridge WM, Rosenfeld RG. Human blood–brain barrier insulin-like growth factor receptor. Metabolism. 1988;37(2):136–40.
Golden PL, Maccagnan TJ, Pardridge WM. Human blood–brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Investig. 1997;99(1):14–8.
Boado RJ, Golden PL, Levin N, Pardridge WM. Up-regulation of blood–brain barrier short-form leptin receptor gene products in rats fed a high fat diet. J Neurochem. 1998;71(4):1761–4.
Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126(2):465–73.
Vitali C, Wellington CL, Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103(3):405–13.
Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J Neurochem. 2002;81(1):203–6.
Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114(1–2):168–72.
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood–brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45.
Uchida Y, Tachikawa M, Obuchi W, Hoshi Y, Tomioka Y, Ohtsuki S, et al. A study protocol for quantitative targeted absolute proteomics (QTAP) by LC–MS/MS: application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood–brain barrier in ddY, FVB, and C57BL/6 J mice. Fluids Barriers CNS. 2013;10(1):21.
Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood–brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013;102(9):3343–55.
Pardridge WM, Eisenberg J, Yang J. Human blood–brain barrier insulin receptor. J Neurochem. 1985;44(6):1771–8.
Pardridge WM, Eisenberg J, Yang J. Human blood–brain barrier transferrin receptor. Metabolism. 1987;36(9):892–5.
Pardridge WM. Receptor-mediated peptide transport through the blood–brain barrier. Endocr Rev. 1986;7(3):314–30.
Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm Res. 1995;12(6):807–16.
Wu D, Yang J, Pardridge WM. Drug targeting of a peptide radiopharmaceutical through the primate blood–brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J Clin Investig. 1997;100(7):1804–12.
Coloma MJ, Lee HJ, Kurihara A, Landaw EM, Boado RJ, Morrison SL, et al. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17(3):266–74.
Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol Bioeng. 2007;96(2):381–91.
Boado RJ, Pardridge WM. Genetic engineering of IgG-glucuronidase fusion proteins. J Drug Target. 2010;18(3):205–11.
Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotechnol Bioeng. 2008;100(2):387–96.
Boado RJ, Hui EK, Lu JZ, Zhou QH, Pardridge WM. Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein. J Biotechnol. 2010;146(1–2):84–91.
Boado RJ, Zhang Y, Zhang Y, Xia CF, Wang Y, Pardridge WM. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood–brain barrier. Biotechnol Bioeng. 2008;99(2):475–84.
Lu JZ, Boado RJ, Hui EK, Zhou QH, Pardridge WM. Expression in CHO cells and pharmacokinetics and brain uptake in the Rhesus monkey of an IgG-iduronate-2-sulfatase fusion protein. Biotechnol Bioeng. 2011;108(8):1954–64.
Boado RJ, Lu JZ, Hui EK, Sumbria RK, Pardridge WM. Pharmacokinetics and brain uptake in the rhesus monkey of a fusion protein of arylsulfatase A and a monoclonal antibody against the human insulin receptor. Biotechnol Bioeng. 2013;110(5):1456–65.
Boado RJ, Lu JZ, Hui EK, Pardridge WM. Insulin receptor antibody-sulfamidase fusion protein penetrates the primate blood–brain barrier and reduces glycosoaminoglycans in Sanfilippo type A cells. Mol Pharm. 2014;11(8):2928–34.
Boado RJ, Lu JZ, Hui EK, Lin H, Pardridge WM. Insulin receptor antibody-alpha-N-acetylglucosaminidase fusion protein penetrates the primate blood-brain barrier and reduces glycosoaminoglycans in Sanfilippo type B fibroblasts. Mol Pharm. 2016;13(4):1385–92.
Boado RJ, Zhang Y, Zhang Y, Xia CF, Pardridge WM. Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood–brain barrier and Abeta fibril disaggregation. Bioconjug Chem. 2007;18(2):447–55.
Boado RJ, Pardridge WM. Brain and organ uptake in the Rhesus monkey in vivo of recombinant iduronidase compared to an insulin receptor antibody-iduronidase fusion pProtein. Mol Pharm. 2017;14(4):1271–7.
Boado RJ, Hui EK, Lu JZ, Pardridge WM. AGT-181: expression in CHO cells and pharmacokinetics, safety, and plasma iduronidase enzyme activity in Rhesus monkeys. J Biotechnol. 2009;144(2):135–41.
Giugliani R, Nestrasil I, Chen S, Pardridge W, Rioux P. Intravenous infusion of iduronidase-IgG and its impact on the central nervous system in children in Hurler syndrome. Mol Genet Metab. 2017;120:S55–6.
Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–3.
Fishman JB, Rubin JB, Handrahan JV, Connor JR, Fine RE. Receptor-mediated transcytosis of transferrin across the blood–brain barrier. J Neurosci Res. 1987;18(2):299–304.
Bickel U, Yoshikawa T, Landaw EM, Faull KF, Pardridge WM. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc Natl Acad Sci USA. 1993;90(7):2618–22.
Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood–brain barrier in mouse. J Pharmacol Exp Ther. 2000;292(3):1048–52.
Lesley JF, Schulte RJ. Selection of cell lines resistant to anti-transferrin receptor antibody: evidence for a mutation in transferrin receptor. Mol Cell Biol. 1984;4(9):1675–81.
Kissel K, Hamm S, Schulz M, Vecchi A, Garlanda C, Engelhardt B. Immunohistochemical localization of the murine transferrin receptor (TfR) on blood–tissue barriers using a novel anti-TfR monoclonal antibody. Histochem Cell Biol. 1998;110(1):63–72.
Boado RJ, Zhang Y, Wang Y, Pardridge WM. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood–brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102(4):1251–8.
Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood–brain barrier. Sci Transl Med. 2013;5(183):183ra57, 1–12.
Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014;6(261):261ra154.
Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81(1):49–60.
Leoh LS, Daniels-Wells TR, Martinez-Maza O, Penichet ML. Insights into the effector functions of human IgG3 in the context of an antibody targeting transferrin receptor 1. Mol Immunol. 2015;67(2 Pt B):407–15.
Zhou QH, Boado RJ, Lu JZ, Hui EK, Pardridge WM. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood–brain barrier in the mouse. Drug Metab Dispos. 2010;38(4):566–72.
Zhou QH, Boado RJ, Hui EK, Lu JZ, Pardridge WM. Chronic dosing of mice with a transferrin receptor monoclonal antibody-glial-derived neurotrophic factor fusion protein. Drug Metab Dispos. 2011;39(7):1149–54.
Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, et al. High transcytosis of melanotransferrin (P97) across the blood–brain barrier. J Neurochem. 2002;83(4):924–33.
Pan W, Kastin AJ, Zankel TC, van Kerkhof P, Terasaki T, Bu G. Efficient transfer of receptor-associated protein (RAP) across the blood–brain barrier. J Cell Sci. 2004;117(Pt 21):5071–8.
Richardson DR, Morgan EH. The transferrin homologue, melanotransferrin (p97), is rapidly catabolized by the liver of the rat and does not effectively donate iron to the brain. Biochim Biophys Acta. 2004;1690(2):124–33.
Regina A, Demeule M, Tripathy S, Lord-Dufour S, Currie JC, Iddir M, et al. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol Cancer Ther. 2015;14(1):129–40.
Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood–brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26(11):2486–94.
Bockenhoff A, Cramer S, Wolte P, Knieling S, Wohlenberg C, Gieselmann V, et al. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase A. J Neurosci. 2014;34(9):3122–9.
Sarko D, Beijer B, Garcia Boy R, Nothelfer EM, Leotta K, Eisenhut M, et al. The pharmacokinetics of cell-penetrating peptides. Mol Pharm. 2010;7(6):2224–31.
Nedelkov D, Nelson RW, Kiernan UA, Niederkofler EE, Tubbs KA. Detection of bound and free IGF-1 and IGF-2 in human plasma via biomolecular interaction analysis mass spectrometry. FEBS Lett. 2003;536(1–3):130–4.
LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C, Sly WS. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci USA. 2004;101(9):3083–8.
Kan SH, Aoyagi-Scharber M, Le SQ, Vincelette J, Ohmi K, Bullens S, et al. Delivery of an enzyme-IGFII fusion protein to the mouse brain is therapeutic for mucopolysaccharidosis type IIIB. Proc Natl Acad Sci USA. 2014;111(41):14870–5.
Boado RJ, Hui EK, Lu JZ, Pardridge WM. IgG-enzyme fusion protein: pharmacokinetics and anti-drug antibody response in rhesus monkeys. Bioconjug Chem. 2013;24(1):97–104.
Pan SD, Zhu LL, Chen M, Xia P, Zhou Q. Weight-based dosing in medication use: what should we know? Patient Prefer Adherence. 2016;10:549–60.
Vergidis P, Avery RK, Wheat LJ, Dotson JL, Assi MA, Antoun SA, et al. Histoplasmosis complicating tumor necrosis factor-alpha blocker therapy: a retrospective analysis of 98 cases. Clin Infect Dis. 2015;61(3):409–17.
Boado RJ, Ka-Wai Hui E, Zhiqiang LuJ, Pardridge WM. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol Bioeng. 2014;111(11):2317–25.
Baldrick P. Safety evaluation of biological drugs: what are toxicology studies in primates telling us? Regul Toxicol Pharmacol. 2011;59(2):227–36.
De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112(8):3303–11.
Boado RJ, Hui EK, Lu JZ, Pardridge WM. CHO cell expression, long-term stability, and primate pharmacokinetics and brain uptake of an IgG-paroxonase-1 fusion protein. Biotechnol Bioeng. 2011;108(1):186–96.
Robinson RC, Radziejewski C, Stuart DI, Jones EY. Structure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimer. Biochemistry. 1995;34(13):4139–46.
Ahmed A, Whitley CB, Cooksley R, Rudser K, Cagle S, Ali N, et al. Neurocognitive and neuropsychiatric phenotypes associated with the mutation L238Q of the alpha-l-iduronidase gene in Hurler–Scheie syndrome. Mol Genet Metab. 2014;111(2):123–7.
Yund B, Rudser K, Ahmed A, Kovac V, Nestrasil I, Raiman J, et al. Cognitive, medical, and neuroimaging characteristics of attenuated mucopolysaccharidosis type II. Mol Genet Metab. 2015;114(2):170–7.
Valstar MJ, Neijs S, Bruggenwirth HT, Olmer R, Ruijter GJ, Wevers RA, et al. Mucopolysaccharidosis type IIIA: clinical spectrum and genotype-phenotype correlations. Ann Neurol. 2010;68(6):876–87.
Shapiro E, King K, Ahmed A, Rudser K, Rumsey R, Yund B, et al. The neurobehavioral phenotype in mucopolysaccharidosis type IIIB: an exploratory study. Mol Genet Metab Rep. 2016;01(6):41–7.
Strolin M, Krageloh-Mann I, Kehrer C, Wilke M, Groeschel S. Demyelination load as predictor for disease progression in juvenile metachromatic leukodystrophy. Ann Clin Transl Neurol. 2017;4(6):403–10.
Boesch S, Nachbauer W, Mariotti C, Sacca F, Filla A, Klockgether T, et al. Safety and tolerability of carbamylated erythropoietin in Friedreich’s ataxia. Mov Disord. 2014;29(7):935–9.
Simmons LJ, Surles-Zeigler MC, Li Y, Ford GD, Newman GD, Ford BD. Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. J Neuroinflamm. 2016;13(1):237.
Chang R, Knox J, Chang J, Derbedrossian A, Vasilevko V, Cribbs D, et al. Blood–brain barrier penetrating biologic TNF-alpha inhibitor for Alzheimer’s disease. Mol Pharm. 2017;14(7):2340–9.
Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, et al. Inflammatory process in Alzheimer’s and Parkinson’s diseases: central role of cytokines. Curr Pharm Des. 2016;22(5):541–8.
Boehme AK, McClure LA, Zhang Y, Luna JM, Del Brutto OH, Benavente OR, et al. Inflammatory markers and outcomes after lacunar stroke: levels of inflammatory markers in treatment of stroke study. Stroke. 2016;47(3):659–67.
Jiang J, Wang ZH, Qu M, Gao D, Liu XP, Zhu LQ, et al. Stimulation of EphB2 attenuates tau phosphorylation through PI3K/Akt-mediated inactivation of glycogen synthase kinase-3beta. Sci Rep. 2015;29(5):11765.
Zhang X, Winkles JA, Gongora MC, Polavarapu R, Michaelson JS, Hahm K, et al. TWEAK-Fn14 pathway inhibition protects the integrity of the neurovascular unit during cerebral ischemia. J Cereb Blood Flow Metab. 2007;27(3):534–44.
Nayak L, de Groot J, Wefel JS, Cloughesy TF, Lieberman F, Chang SM, et al. Phase I trial of aflibercept (VEGF trap) with radiation therapy and concomitant and adjuvant temozolomide in patients with high-grade gliomas. J Neurooncol. 2017;132(1):181–8.
Tisato V, Gonelli A, Voltan R, Secchiero P, Zauli G. Clinical perspectives of TRAIL: insights into central nervous system disorders. Cell Mol Life Sci. 2016;73(10):2017–27.
Funk KE, Mirbaha H, Jiang H, Holtzman DM, Diamond MI. Distinct therapeutic mechanisms of Tau antibodies: promoting microglial clearance versus blocking neuronal uptake. J Biol Chem. 2015;290(35):21652–62.
Ineichen BV, Kapitza S, Bleul C, Good N, Plattner PS, Seyedsadr MS, et al. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017 (in press).
Tran JQ, Rana J, Barkhof F, Melamed I, Gevorkyan H, Wattjes MP, et al. Randomized phase I trials of the safety/tolerability of anti-LINGO-1 monoclonal antibody BIIB033. Neurol Neuroimmunol Neuroinflamm. 2014;1(2):e18.
Sahin C, Lorenzen N, Lemminger L, Christiansen G, Moller IM, Vesterager LB, et al. Antibodies against the C-terminus of alpha-synuclein modulate its fibrillation. Biophys Chem. 2017;220:34–41.
Webster CI, Caram-Salas N, Haqqani AS, Thom G, Brown L, Rennie K, et al. Brain penetration, target engagement, and disposition of the blood–brain barrier-crossing bispecific antibody antagonist of metabotropic glutamate receptor type 1. FASEB J. 2016;30(5):1927–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
William M. Pardridge is a consultant to ArmaGen, and is co-inventor of patents on biologics drug delivery across the blood–brain barrier.
Funding
No funding was received for the preparation of this review.
Rights and permissions
About this article
Cite this article
Pardridge, W.M. Delivery of Biologics Across the Blood–Brain Barrier with Molecular Trojan Horse Technology. BioDrugs 31, 503–519 (2017). https://doi.org/10.1007/s40259-017-0248-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0248-z