Abstract
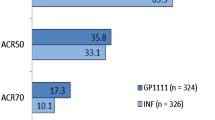
SB2 is a biosimilar of the reference anti-TNF-α antibody infliximab. In May 2015, it was approved in the EU for use in all indications for which reference infliximab is approved, including rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, psoriatic arthritis and psoriasis. It is also approved in these indications in several other countries, including Korea, the USA and Australia. Characterization of SB2 in preclinical studies showed that it is similar to reference infliximab. SB2 demonstrated pharmacokinetic biosimilarity to reference infliximab in healthy volunteers, and clinically equivalent efficacy in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. SB2 was generally well tolerated; the safety and immunogenicity profiles were similar to those of reference infliximab with no additional safety concerns identified. Switching from reference infliximab to SB2 did not impact clinical efficacy, safety or immunogenicity. The role of reference infliximab in the management of autoimmune inflammatory conditions is well established, and SB2 provides an effective biosimilar alternative for patients requiring infliximab therapy.


Similar content being viewed by others
References
European Medicines Agency. Flixabi: EU prescribing information. 2016. http://www.ema.europa.eu/. Accessed 27 July 2017.
Samsung Bioepis. Samsung Bioepis obtains first drug approval in the United States, as the U.S. Food and Drug Administration approves RENFLEXIS™ (Infliximab-abda) across all eligible indications [media release]. 2017. http://www.samsungbioepis.com. Accessed 27 July 2017.
European Medicines Agency. Flixabi CHMP assessment report. 2016. http://www.ema.europa.eu/. Accessed 27 July 2017.
Hong J, Lee Y, Lee C, et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). mAbs. 2017;9(2):365–83.
Choe J-Y, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76:58–64.
Smolen JS, Choe J-Y, Prodanovic N, et al. Comparing biosimilar SB2 with reference infliximab after 54 weeks of a double-blind trial: clinical, structural and safety results. Rheumatology. 2017. doi:10.1093/rheumatology/kex254.
Shin D, Kim Y, Kim YS, et al. A randomized, phase I pharmacokinetic study comparing SB2 and infliximab reference product (Remicade®) in healthy subjects. BioDrugs. 2015;29(6):381–8.
Smolen JS, Choe J-Y, Prodanovic N, et al. Comparable safety and immunogenicity and sustained efficacy after transition to SB2 (an infliximab biosimilar) vs ongoing infliximab reference product in patients with rheumatoid arthritis: results of phase III transition study [abstract no. FRI0162]. Ann Rheum Dis. 2016;75(Suppl 2):488.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Y. Lamb, L. Scott and E. Deeks are salaried employees of Adis/Springer. Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/2C48F0601F5733C1.
Rights and permissions
About this article
Cite this article
Lamb, Y.N., Scott, L.J. & Deeks, E.D. SB2: An Infliximab Biosimilar. BioDrugs 31, 461–464 (2017). https://doi.org/10.1007/s40259-017-0240-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0240-7