Abstract
Objective
To identify how value is defined in studies that focus on the value of molecular testing in cancer and the extent to which broadening the conceptualisation of value in healthcare has been applied in the molecular testing literature.
Methods
A scoping review was undertaken using Joanna Briggs Institute (JBI) guidance. Medline, Embase, EconLit and Cochrane Library were searched in August 2023. Articles were eligible if they reported costs relative to outcomes, novel costs, or novel outcomes of molecular testing in cancer. Results were synthesised and qualitative content analysis was performed with deductive and inductive frameworks.
Results
Ninety-one articles were included in the review. The majority (75/91) were conventional economic analyses (comparative economic evaluations and budget impact assessments) and undertaken from a healthcare system perspective (38/91). Clinical outcomes dominate the assessment of value (61/91), with quality-adjusted life-years (QALYs) the most common outcome measure (45/91). Other definitions of value were diverse (e.g. psychological impact, access to trials), inconsistent, and largely not in keeping with evolving guidance.
Conclusions
Broader concepts of value were not commonly described in the molecular testing literature focusing on cancer. Conventional approaches to measuring the health costs and outcomes of molecular testing in cancer prevail with little focus on non-clinical elements of value. There are emerging reports of non-clinical outcomes of testing information, particularly psychological consequences. Intrinsic attributes of the testing process and preferences of those who receive testing information may determine the realised societal value of molecular testing and highlight challenges to implementing such a value framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Evidence on the value-for-money of molecular testing in cancer is limited. A clear definition of value in this setting is lacking and necessary to inform decision-makers' choices. |
Consideration of broader concepts of value (e.g., beyond health) rarely feature in studies focusing on the value of molecular testing in cancer. |
Greater attention to the psychological consequences, the testing process as a whole, and preferences of those people who receive testing information may influence the value society gets from these modern cancer diagnostic technologies. |
1 Introduction
Molecular testing has a growing role in modern cancer care for diagnosing cancer, predicting responses to genetically targeted drugs, and estimating prognosis. With rapid advancement in the field of precision oncology, the presence of molecular testing in clinical guidelines and demand for molecular testing continues to rise [1, 2]. As is the case for all technological advances in health, it is vital to understand how, where, when and to whom molecular tests provide value for money (i.e., in the economic sense, the necessary evidence base for the quantification of the incremental costs relative to the incremental outcomes). Health technology assessment (HTA) across the technology life cycle [3]—from molecular testing research and development, regulatory approval, pricing and reimbursement, to optimal clinical implementation—requires robust, readily communicated evidence on value to ensure optimal and equitable patient care.
However, existing evidence available to decision-makers on the costs and outcomes of molecular testing in cancer is limited by methodological challenges, including the lack of an agreed definition for value [4] and inconsistent approaches to quantifying value [5,6,7,8,9,10,11,12]. Terkola's editorial [4] summarised the key challenge in economic evaluation in precision medicine: A lack of high-quality data on costs and health outcomes underscored by the lack of a consensus definition of value.
Conventional approaches to measuring incremental costs and incremental benefits (e.g., the commonly used cost-utility analysis (CUA) with quality-adjusted life-year (QALY) [13, 14]) have been criticised as not fit for purpose. This is especially the case in fields like precision oncology where diverse short- and long-term outcomes from cancer genomics information can benefit diverse stakeholders and resource utilisation subsequent and consequent to testing influence cost-effectiveness [10, 15].
Recognition of a healthcare intervention's non-health benefits (beyond what can be presented by a QALY) hallmarks the contemporary broadening conceptualisation of value in healthcare [16] and the consequent attention to societal perspectives of value. For healthcare decision-making to represent preferences of society, requisite evidence generation must capture views on health and non-health outcomes [17, 18] from a broad stakeholder group [16]. Actively involved in this discussion, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) published their 2018 “value flower” [17], which summarises ordinarily underappreciated elements of value. The same narrative has extended to precision medicine and diagnostics, where recent health economics literature has invited a shift towards a more comprehensive definition of value [7]. For example, the Office of Health Economics (OHE) White Paper 'Improving the HTA of Complementary Diagnostics' called for "a more comprehensive perspective to include the less tangible benefits [of complementary diagnostics] [19]. Similarly, the Institute for Clinical and Economic Review’s (ICER) 2020–2023 Value Assessment Framework report suggested that "important modifications" to the evaluation of diagnostic tests are required to account for the distinct uses of diagnostic information, including explicit consideration of "other benefits, disadvantages and contextual considerations” [20]. For molecular testing in cancer, where assessment has previously focused heavily on analytical validity, clinical validity and clinical utility [21], this means a shift towards the generation of evidence that includes non-health benefits such as personal utility [22, 23] and process outcomes [15]. These more ‘novel’ elements of value [17, 18] are not yet well integrated into healthcare decision-making (e.g., in Australia or the UK [24, 25]), likely biasing previous resource allocation decisions. Similarly, investment in innovation may not have been well aligned with future societal value for money [26]. A recent systematic review on the cost-effectiveness of companion diagnostics for targeted cancer therapies [27] concluded that studies fail to observe factors that influence value beyond costs and sensitivity/specificity.
To what extent studies that focus on costs and outcomes of molecular testing in cancer reflect this shift toward a broader conceptualisation of value and the representation of value from a range of perspectives is unclear. Issues relating to social and health equity are pertinent, especially in Aotearoa New Zealand where there are stark and unjust existing inequities in health outcomes for indigenous Māori and Pacific peoples (e.g., Māori are 20% more likely to develop cancer than non-Māori and twice as likely to die from cancer [28]), and germane to molecular testing in cancer where access is notoriously variable. As such, incorporation of equity or viewpoints of indigenous communities in value definitions warrant attention.
This scoping review aims to identify how value is defined in studies that focus on the value of molecular testing in cancer with a key objective to map use of the more ‘novel’ elements of value.
The review sought to answer the following questions:
-
1.
How is value defined in studies that focus on the value of molecular testing in cancer?
-
2.
What elements (particularly novel elements) are considered?
-
3.
What is the range of evidence that focuses on value (particularly novel elements) of molecular testing in cancer?
2 Methods
2.1 Choice of Review Methodology
Since the main purpose of the review was to identify and map definitions from within the available evidence, a scoping review was selected as the most appropriate approach [29, 30]. The methods were informed by JBI guidance [30] and documented in an a priori protocol (review topic registered to the Open Science Framework;Footnote 1 the protocol is available through the corresponding author). Changes to the protocol, in line with the iterative nature of scoping reviews described in JBI’s population, concept and context framework guidelines [30], are described in Sects. 2.2.2, 2.3 and 2.4. The review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [31].
2.2 Scope and Eligibility Criteria
The Participant, Concept, Context approach for developing eligibility criteria was adopted.
2.2.1 Participant
There were no eligibility criteria relating to participants.
2.2.2 Concept
The concept being explored was the definitions of value used in studies focusing on the value of molecular testing in cancer. Value is a ubiquitous but inconsistently used term across healthcare and takes different meanings within clinical and economic domains. Common to most definitions of value are a comparison of resources used (costs) relative to outcomes (consequences or benefits) with respect to perspective and decision-making context. As such, ‘focusing on value’ was defined as the attention given to estimating costs, outcomes, or contextual considerations (referred to collectively as the determinants or elements of value). This was regardless of whether an explicit definition of value is given or implicitly inferred by reporting specific elements.
Consistent with reference to molecular testing across disciplines researching precision oncology (e.g., clinical, health economic, biosciences), the definition for molecular testing was kept broad to include its categorisation by clinical use (e.g., pharmacogenomics test, companion diagnostic), economic properties (e.g., complementary diagnostic), and technology type (e.g., immunohistochemistry, DNA sequencing, whole genome sequencing (WGS)). It is considered a process (as opposed to an event discrete in time or place), so a range of diagnostic or care pathways (e.g., cancer screening, risk stratification, treatment selection), including a molecular testing component, were considered relevant, provided there was sufficient focus on reporting the value of molecular testing. ‘Cancer’ was defined as solid organ or haematological malignancy in humans and not limited by tumour type, morphology or stage. Animal studies, in silico studies, and studies focused on pre-implantation diagnosis were excluded.
2.2.3 Context
Articles from any country and research discipline were eligible.
2.3 Sources of Evidence
The review sought to identify primary research studies, including experimental, observational and economic modelling studies published in the past 5 years. This aligned with the publication of the ISPOR’s Special Task Force’s recommendations on value frameworks [16].
Economic evaluation studies (comparative economic evaluations and budget impact assessments) were included, as well as qualitative, quantitative or mixed methods preference research, which a preliminary review [see Online Supplementary Materials (OSM)] had signposted as key evidence reporting information on personal utility.
Given the interest in identifying novel elements, the review sought to include studies estimating costs relative to outcomes, or costs or outcomes provided that at least one of these was non-clinical. Exclusion criteria were developed to avoid the large body of established literature reporting on molecular testing diagnostic accuracy, predictive or prognostic value, usually without comparison to costs. Although within scope, these types of studies were not included because the preliminary review demonstrated that diagnostic accuracy is an already well-recognised determinant of value for molecular testing in cancer, and inclusion would detract from mapping broader references to value. Similarly, we excluded the literature on microcosting of molecular testing in cancer covered in a recent systematic review [32].
Reports from health technology organisations in grey literature were considered for inclusion. Editorials, perspective pieces, reviews, abstracts, conference proceedings and protocols were excluded. The review was limited to full text articles published in English.
2.4 Search Strategy
The search strategy followed JBI’s three-step approach [33], starting with a limited search (Appendix I) in Embase. The preliminary review identified search terms to support the exclusion of diagnostic accuracy studies (e.g., ‘analytical utility’ and ‘positive predictive value’) and to increase the chance of including studies with a focus on non-clinical elements. Informed by analysis of relevant index terms and descriptors in the titles and abstracts of studies identified through the initial search (OSM), a detailed search strategy was developed with the assistance of a University of Auckland research librarian (Appendix II). One reviewer (AM) translated the search strategy of keywords, MeSH and Embase subject headings to undertake searches across Medline, Embase, EconLit and Cochrane Library on 15 August 2023. Grey literature was searched on websites from organisations listed by the International Network of Agencies for HTA. The reference lists of the most relevant articles were manually searched for additional potentially eligible studies [34].
2.5 Study Selection
Articles were imported into Covidence (Veritas Health Innovation, Melbourne, VIC, Australia) via EndNote X9.3.3 (Clarivate Analytics, PA, USA). Duplicates were removed before entering two-phased screening. Two reviewers (AM and FSG) independently screened titles and abstracts of articles identified through database searching for relevance to the inclusion criteria, and then the full-texts of articles deemed relevant were retrieved and examined for evidence pertaining to the review questions and outcomes of interest. During the two-phased screening, iterative eligibility criteria refinement was made through reviewers' discussions. These iterations aimed to increase the identification of novel value elements and respond to the ambiguity in determining the degree of focus on value of molecular testing (as opposed to the value of a molecularly informed intervention), i.e., the initial definition of ‘focus on value’ proved insufficiently detailed for the attention of this review to identify more novel value elements. Key eligibility criteria refinements were:
-
Include studies with focus on how testing procedures are implemented, acknowledging that these represent value elements influencing the realised value of molecular testing in cancer.
-
Include studies even if unclear whether the molecular testing or the molecularly informed intervention has the greater focus, provided there is a clear description of a molecular testing component within a studied intervention.
-
Exclude studies that primarily aim to estimate the impact of changing a testing eligibility criterion (e.g., expanding the eligible population for population screening where the screening intervention includes molecular testing).
Conflicts arose in the title and abstract and full-text screening processes, as the central concept of focus on value of molecular testing in cancer is not well defined or articulated in the literature. Reasons for excluding full-texts were documented and discrepancies resolved through discussion with two other authors (PL and MW) if necessary, with a return to the definition of value outlined in this article’s introduction.
2.6 Data Extraction
One reviewer (AM) extracted study characteristics and information relating to review questions from the included articles using a pre-defined standard data extraction table developed in Microsoft Word (Appendix III) and translated into Covidence. The extracted information included data pertaining to bibliographic data, information relating to the molecular test, analysis methodology, and value definitions. For value definitions, explicit or implicit definitions were extracted in textual form, with implicit definitions considered the mention of a cost, outcome or contextual consideration from outside article discussions. Value outcome measures (e.g., QALYs) were extracted.
Value elements were first mapped to a binary yes/no for the inclusion of non-clinical outcomes. Non-clinical outcomes were mapped against the ‘non-core’ elements of value of health technologies as depicted in ISPOR Special Task Force’s Elements of Value [17] (the earliest and most well-known value framework), and the Office of Health Economics (OHE) ‘non-traditional’ elements of value for complementary diagnostics [19] (Fig. 1). ISPOR’s Elements of Value is the earliest and most well-known of the value frameworks. The OHE framework was selected because it was developed in a different country (UK) and specifically for diagnostics. Textual information of novel costs, benefits or contextual considerations was also recorded. Two reviewers (AM and FSG) piloted the extraction form and modified iteratively to ensure consistency and accuracy. Alterations included clarifying extracted date as the earliest date of publication and country of publication as country of ‘focus’. Some extraction fields proved too subjective (e.g., decision-making context and clinical application) and not suitable for descriptive frequency counts. Similarly, molecular test technology was not clearly defined within studies and so could not be sub-categorised for analysis. Ambiguity of interpretation of study reference to ISPOR or OHE value elements was resolved through discussion and returning to the Special Task Force paper and related references [16, 35, 36]. For example, in the context of cascade screening of relatives it was agreed that outcomes for modelled relatives did not fulfil the definition of family spillover, which relates to the impact that illness can have on informal/unpaid caregivers/family members [35]. Value of knowing and value of reduction in uncertainty were considered mutually inclusive [19, 37, 38]. The second reviewer (FSG) cross-checked completeness and accuracy of the extracted data of 10% of the articles where clinical outcomes only were identified and 10% of the articles where novel elements were identified (to weight the cross-checking onto articles of greater focus for analysis).
Selected frameworks for mapping of non-clinical outcomes. Left: ‘Non-core’ elements of value of health technologies as depicted in International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Special Task Force’s Elements of Value [17]. Note: Green circles: core elements of value; light blue circles: common but inconsistently used elements of value; dark blue circles: potential novel elements of value; blue line: value element included in traditional payer or health plan perspective; red line: value element also included in societal perspective. Right: Office of Health Economics ‘non-traditional’ elements of value for complementary diagnostics [19]. Note: Light grey circle: traditional elements of value as considered by health technology assessment (HTA); dark grey circle: expanded value framework (elements not traditionally considered/measured); green line: value from health system perspective; red line: value also included in societal perspective
2.7 Data Synthesis
Descriptive statistics using frequency counts were conducted for study characteristics information to address the mapping of available evidence that focuses on value of molecular testing in cancer objective. Value outcome measures were categorised as ‘conventional economic’ (QALYs, life-years (LYs), disability-adjusted life-years (DALYs), other natural units or a monetary measure (e.g., $)), or ‘non-economic’ and presented with descriptive statistics. A descriptive qualitative context analysis approach was used to collate the extracted value definitions textual data [33]. The degree to which the available literature has addressed previously proposed novel value elements was made deductively using frequency counts of references (excluding cites in the Discussion section) to value elements from the selected OHE and ISPOR model frameworks. Collaborative and iterative development of an inductive framework to further synthesise the evidence pertaining to novel value definitions was required for those outcomes not readily mapped to the OHE and ISPOR frameworks. The reviewer who had performed the main extraction (AM) undertook open-coding using NVivo (v 14.23.2, QSR International). AM shared these thoughts with the other authors and incorporated their reflections into developing initial categories to describe the extracted data [33]. After re-presentation of these categories and further iterative modification, a coding framework for organising the data was agreed.
Data are summarised according to review questions using tables, figures and narrative synthesis.
3 Results
3.1 Search Results
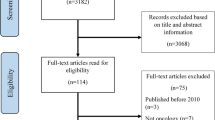
The four database searches returned a total of 1629 results and five articles were identified through grey literature. After removing 556 duplicates, titles and abstracts of the remaining 1086 articles were screened for relevance to the inclusion criteria. Screening resulted in the exclusion of 923 irrelevant articles, and the remaining 163 progressed to full-text review. Of these, 72 were excluded for reasons documented in the PRISMA diagram (Fig. 2). Eight relevant articles were identified through hand-searching. In total 91 articles met eligibility criteria for this review.
3.2 Characteristics of Included Articles
The main article characteristics are depicted in Table 1. These comprised 75/91 (82.42%) conventional economic analyses (with the majority cost-effectiveness (CEA) or CUA) and 16/91 (17.58%) other study types (e.g., mixed methods or qualitative studies).
Most molecular testing scenarios considered within articles were based in the USA, followed by Australia, Canada, the UK, Spain, then China (Fig. 3). The most examined perspective was the healthcare system, followed by third-party payer. When accounting for studies that considered multiple perspectives, the 91 articles included 102 perspectives, including 12 patient, nine societal, and five public perspectives.
There were no observed changes over time in study characteristics (not presented).
3.3 How is Value Defined?
3.3.1 Inclusion of Non-clinical Outcomes
A minority (30/91, 33.0%) of articles considered non-clinical outcomes. Two-thirds of articles mentioned clinical outcomes alone. Of the conventional economic analyses, the proportion of studies considering non-clinical outcomes was even less (61/75, 81.3% vs. 14/75, 18.7%). There has been a recent observed increase in the inclusion of non-clinical outcomes within conventional economic analyses (Fig. 4).
3.3.2 Outcome Measures
Most studies used conventional economic outcome measures to define value; in these economic evaluations cost per QALY was the sole outcome measure in half (45/91) of included articles. Several studies (17/91) used combinations of conventional economic outcome measures to report value, predominantly QALYs or LYs with a natural unit. Five articles presented LYs, and one study presented DALYs alone.
In the 16 articles that were not conventional economic analyses (e.g., qualitative studies, discrete choice experiments (DCEs)), the most common sole outcome was willingness to pay (WTP) (5/16, 31.3%). Other outcomes included attitudes towards, experiences of, interest in, knowledge and understanding of, and preferences for implementation strategies of molecular testing in cancer.
3.3.3 Mapping of Value Outcomes to ISPOR Non-core Value Elements and Office of Health Economics (OHE) Non-traditional Value Elements
Value of knowing (ISPOR) and reduction in uncertainty (OHE) were commonly identified novel elements, identified 22 times within included articles. Other ISPOR non-core value elements were also identified: equity (n = 10 articles), productivity (n = 9), value of hope (n = 6), family spillover (n = 6), severity of illness (n = 5), scientific spillover (n = 3), insurance value (n = 2) and real option value (n = 2). The OHE non-traditional value elements were identified less often: value of hope (n = 6 articles), cost-savings outside of the health system (n = 3), scientific spillover (n = 3), insurance value (n = 2) and real option value (n = 2). This mapping of value outcomes is shown in Fig. 5.
3.3.4 Non-clinical Elements of Value
Numerous novel (not conventionally reported clinically related) elements were identified through this review (a proportion of which could be mapped to OHE and ISPOR frameworks). It became evident, through the inductive approach, that these could be grouped into: (1) attributes of tests highly relevant to molecular testing in cancer and not ordinarily considered in conventional economic analyses (herein referred to as ‘novel test attributes’); (2) attributes of the clinical testing indication; (3) intrinsic attributes of people involved with testing (stakeholders) (herein referred to as ‘people attributes’); (4) non-clinical outcomes of testing; and (5) clinical outcomes of testing highly relevant to molecular testing and not ordinarily considered in conventional economic analyses (herein referred to as ‘novel clinical outcomes’). These five categories are presented within an overall framework (Fig. 6). While some elements may fit more than one category, each element was placed into one category determined to be the ‘best-fit’ for the purposes of presenting these data.
Overall framework demonstrating grouping of novel value elements identified from included articles developed through inductive analysis. Note: Equity was implicitly considered across other groups, with the majority of examples relating to people attributes (examination of the heterogeneity of preferences for, experiences of, or willingness to pay for molecular testing), novel test attributes, and indication attributes. Elements of value within these five groups were considered at different levels: individual, family, community, health system and society
3.3.4.1 Novel Test Attributes
Many articles sought to examine attributes intrinsic to properties of the molecular testing. These included reference to the clinical purpose for the test (e.g., screening, companion diagnostic) [39] with suggestion—especially from the patient perspective—that tests informing of treatment choice are of greater importance than others. For example, in one article “Finding a new treatment was the overriding motivation…[authors comment that]…identification of a germline variant were generally seen as ancillary” [39]. While breadth of test result information was considered in another study that examined patient and clinician preference for receiving test results with or without germline information [40].
Others examined more operational attributes relating to the test process, such as biopsy requirements (e.g., number [41], ability to use historical tissue [42] or nature/invasiveness [40] and the related issue of tissue preservation [40]), role/extent of healthcare professional (HCP) involvement for pre-test counselling or return of results [43], format for return of results (e.g., patient-mediated vs. provider-mediated [43]), and geographic location of tissue acquisition or tissue processing, data interpretation, storage or return of results [40, 42]. A frequently recognised element was the turn-around time (linked to the patient-centred wait-time for results) [40, 41, 44,45,46]. Related to this, one article mentioned that batching tests for processing can directly, but not necessarily predictably, influence turn-around and wait-times [45].
3.3.4.2 Indication Attributes
Another category evident from the included articles related to attributes of the clinical indication for testing. These included clinical seriousness or urgency for decision-making. For example, [study participant comments] “Why did I have to wait until treatment options were exhausted before being eligible?” and [authors comment that] “A subgroup of participants reported…they were running out of…options…a ‘ticking time-bomb’ with death increasingly close” [39]. One paper considered the rarity of a clinical condition [47].Footnote 2
3.3.4.3 People Attributes
Many articles brought attention to attributes that are intrinsic to persons, often presented as heterogeneity of preferences for molecular testing. These most related to the person(s) undergoing testing and included socioeconomic (e.g., ethnicity, rurality, social isolation, income) and clinical (e.g., current health status, reproductive characteristics, competing health priorities, family history [48]) factors. For example, one study reported a participant’s perception of gene testing as “unnecessary because of current healthiness” [42].
Numerous intrinsic factors can be grouped as psychological preferences, perceptions, and behavioural traits. This included sense of desperation [39], optimism [48], hopefulness [44, 47], degree of trust in oncologists or science[39], and aversion or tolerance to uncertainty [44, 47, 49]. Regier et al.’s DCE estimated public preferences and demand for molecular testing that informs treatment choice by outcomes in context of uncertainty and found that “respondents identified uncertainty as among the most important considerations when providing precision medicine that is valued by patients” [49]. Five studies looked at individual’s perceived risk for, or severity of, the cancer type of interest [39, 43, 47, 50, 51]. High health monitoring behaviour was identified in one study as a determinant of WTP for breast cancer susceptibility testing [48]. ‘Self-efficacy’, defined as confidence in one's ability to cope (e.g., with risk prevention strategies) with actionable or non-actionable results was examined in a study investigating preferences and WTP for WGS amongst cancer patients and their families [47]. Another article mentioned the perceived caution or enthusiasm of HCPs towards experimental treatments as an element influencing their experience of genomic and personalised paediatric oncology [52].
Relational elements were considered in some studies. These included individual’s sense of responsibility to their family to obtain information that could influence their health [47], likelihood to recommend testing to others [50], having a locus of control highly attributed to powerful others [48], nature of relationship with HCPs [52], and connectedness with the healthcare system [43].
Specific skills and knowledge were examined, including at individual and system levels. The most identified skillset for individuals undergoing testing was health (or more specifically genomic) literacy, which was considered in seven articles [39, 42,43,44, 53,54,55]. Relatedly, two studies examined heterogeneity of WTP for molecular testing by education level [47, 48]. Other specific skills examined included quality of an individual’s communication skills to enable them to share results, as well as their ability to process complex information or manage negative emotions [43].
Some studies gave attention to the skills and knowledge of test providers. These identified genomic literacy [53], handling of uncertainty [49], and managing patient expectations [52] as key skills necessary for clinical implementation of molecular testing.
Healthcare system-level attributes were also identified. Examples relating to system strategy included the degree of future focus, acceptance of uncertainty, or ability of a health system to adapt to evolving evidence [49, 56].
3.3.4.4 Non-clinical Outcomes
The majority of novel consequences of testing identified from the included articles are categorised here as non-clinical outcomes of molecular testing and subcategorised into psychological impacts for those undergoing testing and non-clinical common good outcomes.
Psychological impacts for those undergoing testing The identified psychological outcomes of the person(s) undergoing testing relate to the value of knowing. For example, decreased worry/anxiety [54, 57], reduced uncertainty [48], greater ability to plan (e.g., family planning [48]), or the value in hope of what the results may lead to [39, 44, 52]. One study identified outcomes from knowing as “empowering” [authors comment], providing individuals with an explanation for their diagnosis, absolving the responsibility for causing their own illness, or validation for previous medical decisions [57]. “I [study participant comments] just feel like a weight’s been lifted off my shoulders …I was worried about ovarian cancer but I’m not now. I feel really happy about that” [57].
In CEAs/CUAs, attempts to quantify the utility of the value of knowing were seen. One study assumed that after receiving a negative test result a woman’s utility increases to that of healthy women [58]. Another approach was considered within scenario analyses [59, 60]. For example, Chandler et al. used utility weighting to assess the possible reassurance or worry about cancer recurrence [59].
Illustrating the potential disutility of knowing, included studies considered increased worry and anxiety [54], feeling negative about lack of actionable findings or disutility from receiving an uncertain result [61], and fear of stigmatisation and guilt [62]. An identified specific concern was privacy related to who has access to testing results [39] and implications for insurance [51, 54]. “[If] the final conclusive tests are negative and everything’s fine and then you put your people through two weeks of total terror for not a whole lot of benefits” [study participant comments] [51] “As long as we have a ‘healthy’ political system, this information will probably be used for good. Unfortunately, history shows that humans are able to use such information to harm others” [study participant comments][54].
One article highlighted the heterogeneity of value of knowing, with opposition to obtaining molecular testing information on risk “Leave it in God’s hands and if I get cancer, that’s the card I was dealt” [study participant comments] [51]. In contrast, another article included the disutility of not knowing after declining a test [63]. Another described risk of false reassurance [64].
Non-clinical common good outcomes An extension of the value of knowing beyond the person(s) undergoing testing was identified in some articles. McMullen et al. gave mention to value of knowing to a whole community and future generations [51]. Relatedly, two articles examined scientific spillover [39, 44].
Implications of time spent undergoing testing, molecularly informed interventions, or with illness were frequently described as productivity gain or loss. These were identified in nine studies [56, 59, 62, 64,65,66,67,68]. An additional two studies linked this to time costs to family [62, 69].
Another wider outcome was non-clinical costs-savings such as consideration of informal care costs [62].
3.3.4.5 Novel Clinical Outcomes
While identifying clinical outcomes was not the objective of this scoping review, some elements were identified as particularly relevant to molecular testing in cancer. They are listed here for their potential novelty because they are not conventionally considered in value assessments.
-
Magnitude and nature of impact of molecularly informed management on quality of life or life expectancy.
-
Availability, accessibility, and cost of molecularly informed management.
-
Access to clinical trials.
-
Strength of evidence in the face of evolving evidence base.
One article examined preferences for testing dependent on the chance of an actionable outcome [40].
The concept that molecular testing in cancer’s value is directly connected to access to molecularly informed management was evident in several articles. These considerations spanned cost [52], public/‘reimbursed setting’ availability [40], systemic racism [51], and access to clinical trials [45]. The strength of evidence for intervention for molecularly informed intervention was represented by factors influencing clinician’s decision-making on the basis of scientific evidence [52] and extent of medical agreement on changing care [49].
4 Discussion
4.1 Summary of Findings and Attention to Broadening Conceptualisation of Value
This scoping review sought to identify how value is defined in studies that focus on the value of molecular testing in cancer and to map the literature addressing this topic. Because of the diversity in molecular testing technologies and applications, this review was not limited to any molecular testing type or clinical setting, instead taking a broad definition to molecular testing in cancer. This review pragmatically defined ‘focus on value [of molecular testing in cancer]’ to answer the review questions. The scoping review was designed to strike a balance between identifying the most common approaches to measuring value for money and identifying novel costs or novel benefits at the same time. As such, the review included studies focusing on costs relative to outcomes (to map trends of this core body of research) and studies that focused on describing at least one novel cost or outcome. Molecular testing in cancer diagnostic accuracy was not the focus of this review, given an extensive established body of literature and general acceptance that this is part of value assessment of molecular testing in cancer [70]. A wide range of value definitions was identified, but the overriding focus was on molecular testing's clinical outcomes, with 30/91 (33.0%) studies considering non-clinical outcomes. Attention to non-clinical outcomes was primarily seen within qualitative research, for example, in preference studies, but a minority of conventional economic evaluations also included non-clinical outcomes. The data from 2022 suggest that non-clinical outcomes may be increasingly reported within conventional economic evaluations.
This review used two published value frameworks, namely ISPOR Special Task Force’s Elements of Value [17] and the OHE’s ‘non-traditional’ elements of value for complementary diagnostics [19], to examine the degree to which existing literature has included contemporarily recognised novel value elements. These frameworks have been designed for general pharmaceuticals (ISPOR) and (non-cancer specific) companion diagnostics (OHE), and so were not designed to accommodate the uniqueness of molecular testing in cancer. For the included molecular testing in cancer studies, the value of knowing and the comparable value of reduction in uncertainty were the most featured, followed by equity and productivity. There was little reference to the remaining six ISPOR and four OHE novel elements, suggesting that some of these previously described value elements may not be relevant to molecular testing in cancer, or alternatively, there remain hurdles to operationalising the assessment and measurement of these novel elements [35].
The review identified other non-clinical outcomes and contextual considerations suggesting that molecular testing in cancer likely requires a bespoke approach. For these value elements not ordinarily considered in conventional economic analyses, and therefore considered novel, an inductive approach to analysis resulted in grouping of the elements into five categories representing intrinsic and extrinsic factors: (1) novel test attributes; (2) indication attributes; (3) people attributes; (4) non-clinical outcomes of testing; and (5) novel clinical outcomes. The final category was included as despite pertaining to clinical outcomes, these were considered highly specific to molecular testing in cancer, or previously underappreciated. This inductive framework identified a triad of intrinsic test properties, intrinsic clinical indication properties, and attributes of people (stakeholders) involved with testing as contextual considerations likely to influence the realised value of molecular testing in cancer. The two remaining categories relate to consequences of testing information with particular emphasis on psychological utility or disutility of knowing and spillover effects beyond the individual undergoing testing.
4.2 How did Equity Feature? and Other Gaps
There were notable gaps identified from the examined literature. Given the important focus on addressing inequities in cancer outcomes, especially for indigenous populations, this review aimed to identify elements relating to equity or viewpoints of indigenous communities within value definitions. Equity was not explicitly described within value definitions of included articles. Even when equity was considered broadly (recognizing that different people with different levels of advantage require different approaches and resources to get equitable health outcomes [71]), it rarely featured. Where it did feature, equity was implicitly considered, with the majority of examples relating to examination of the heterogeneity of preferences for, experiences of, or WTP for, molecular testing, which in the inductive framework is mostly embedded within the intrinsic attributes of people category.
Another category where equity was implicitly considered was within test attributes with regards to test process. Specifically, articles identified the barriers to testing (e.g., financial, complexity of navigating health system in context of health literacy or racism) as well as the inequitable distribution of these barriers. Finally, equity is implicitly mentioned in the clinical indication for testing category where attention to rarity was mentioned. This interpretation of equity has been previously used in the context of value assessment with the connection between rarity and unmet need aligning with DrugAbacus’ definitions [72]. There is debate on whether rarity of disease warrants consideration as an independent determinant of value. The National Institute for Health and Care Excellence (NICE) has not yet included rarity of disease as a modifier, commenting on the “complex interactions between the severity of the diseases, current diagnostic and treatment options, clinical knowledge, research and development, and health service design and delivery” [73]. The related issue of rarity of molecular testing results is so far unexplored.
4.3 What do the Observed Trends Suggest is the Future Direction of Value Assessment of Molecular Testing in Cancer?
Value assessment frameworks for health technologies have received heightened attention in the past decade [16, 74]. Aside from a few exceptions designed for diagnostics (e.g. [19, 75]), these have mostly been for pharmaceuticals. ISPOR’s value flower was developed for drugs. Unsurprisingly, it was the flower’s value of information petal that featured most prominently in this review. The earliest descriptions of this value element, now more than 30 years ago, related to diagnostics where a test is valued for the prognostic information it provides apart from its effect on patient management [38]. The attention to this element suggests that further developments toward more holistic molecular testing in cancer value assessments could take direction from the emerging approaches to broaden the value assessment for diagnostics, especially consideration of the value gained from reduction in uncertainty. On the other hand, identified novel elements emerging from this review (consideration of rarity, observed heterogeneity of preferences based on genomic literary, issues relating to variants of uncertain significance, and likelihood of individuals to share information on germline findings with family) are less specific to diagnostics and more linked to precision medicine. As such, value assessment frameworks developed for precision medicines (e.g., value assessment of orphan drugs for rare diseases [76]) may provide more helpful guidance. Another alternative is that development of value assessment tools for molecular testing in cancer forges its own path, although arguably this is not the most practical or efficient approach for health technology assessors [35].
4.4 Implications for Implementation Strategies—Observed Novel Elements Signpost Where Realised Value Could be Optimised
The attention to attributes of the entire testing process and preferences for these attributes suggests that how molecular testing in cancer is implemented likely influences its realised value. Importantly, this signals the need for greater attention to where cost-effectiveness of molecular testing in cancer can be improved with changes in access to genetic counselling, genomics education, etc. One of the included articles emphasised this, highlighting the importance of culturally and linguistically appropriate testing services [42].
4.5 Limitations of the Scoping Review
First, this review may have missed non-English studies that have focused on value of molecular testing in cancer, particularly grey literature, and studies without full text on the net (i.e., FUTON bias [77]). Second, search strategy could not incorporate all studies exploring potentially relevant determinants of value, especially the heterogenous body of implementation and preferences literature for which keywords are not consistent. This would include a large body of qualitative literature on genomic testing in cancer (e.g., information preferences [78,79,80]), which was not identified in this review because the search strategy required the term ‘value’ (or a related word) in an article’s title. Findings from this review should be considered alongside other molecular testing literature including that focusing on analytical validity, clinical validity and clinical utility. Third, the eligibility criteria adopted could not overcome the inherent ambiguity of the ‘focus on value of molecular testing in cancer’ criterion. The extent to which molecular testing comprises a healthcare intervention is not always clear, and the degree to which a study was focused on value proved very subjective. Fourth, the mapping to emerging guidance was limited to ISPOR and OHE, with potential that review conclusions would have differed had alternative deductive frameworks, for example, Augustovski et al.’s Value framework for the Assessment of Diagnostic Technologies [81], been selected, especially since ISPOR is not designed for diagnostics.
4.6 Next Steps in Understanding Value of Molecular Testing in Cancer—Exploring Elements of Value and Approaches to Their Measurement
The dominance of clinical value elements and prevalence of use of the QALY concluded from this review demonstrates the tendency for studies estimating molecular testing in cancer 's value to focus on quantifiable and commensurable elements. Combined with the fact this review identified no multi-criteria decision analyses, this suggests a gap in the literature with respect to trialling methods that more deliberatively assess value, like the approach increasingly seen in the HTA of pharmaceuticals [82].
It is likely that the design of studies that focus on the value of molecular testing in cancer are dictated by the market they are trying to receive reimbursement from. For jurisdictions where the decision and reimbursement of molecular testing and targeted medicines or preventative strategies may be independent of each other (e.g., different decision-making bodies), evidence may be interpreted without due attention to complementarity [18].
A next logistical step is the further exploration of the non-clinical elements of value important to the range of relevant stakeholders, with attention to the complex interplay and joint influences of psychological consequences of knowing, value of reduced uncertainty, and spillover outcomes in the context of precision oncology’s heterogeneity of outcomes. Once these are better understood the field can move onto the quantification and commensuration of the conventional and novel elements of value. It is not known whether the identified novel elements are reflective of indigenous peoples’ values as this research has not been undertaken, highlighting a priority focus for future research. Also, while this is a scoping exercise to map how value is defined for molecular testing in cancer, our review findings suggest that once elements of value are identified, data for these must be collected.
4.7 Conclusions
Molecular testing in cancer has increased clinical attention, necessitating robust approaches to value assessment to support optimal and equitable implementation. Published studies that focus on the value of molecular testing in cancer highlight the lack of an agreed definition of value in this setting. Ongoing research is warranted to clarify how value is defined in a way that accounts for molecular testing as a process, and with a breadth of outcomes through an equity lens, including psychological benefits and benefits beyond the tested individual.
Notes
OSF Registries | Definitions and elements of value used in the assessments of value in molecular testing in cancer: A scoping review.
(Perceived) severity of condition has been allocated to people attributes because it was largely documented as a perception rather than an objective clinical descriptor.
References
The Business Research Company. Oncology molecular diagnostics global market report 2022. 2022. https://www.businesswire.com/news/home/20220830005582/en/Oncology-Molecular-Diagnostics-Global-Market-Research-Report-2022-PCR-ISH-INNAT-Chips-Microarrays-Sequencing-Mass-Spectroscopy-TMA-Analysis-Forecasts-2016-2021-2021-2026F-2031F---ResearchAndMarkets.com. Accessed 19 Nov 2023.
Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:497–530.
Regier DA, Pollard S, McPhail M, Bubela T, Hanna TP, Ho C, Weymann D. A perspective on life-cycle health technology assessment and real-world evidence for precision oncology in Canada. NPJ Precis Oncol. 2022;6(1):76.
Terkola R, Antoñanzas F, Postma M. Economic evaluation of personalized medicine: a call for real-world data. Eur J Health Econ. 2017;18(9):1065–7.
Akhmetov I, Bubnov RV. Assessing value of innovative molecular diagnostic tests in the concept of predictive, preventive, and personalized medicine. EPMA J. 2015. https://doi.org/10.1186/S13167-015-0041-3.
Burris HA, Saltz LB, Yu PP. Assessing the Value of next-generation sequencing tests in a dynamic environment. Am Soc Clin Oncol Educ Book. 2018:139–146.
Faulkner E, Holtorf AP, Walton S, et al. Being precise about precision medicine: what should value frameworks incorporate to address precision medicine? A report of the personalized precision medicine special interest group. Value Health. 2020;23:529–39.
Marshall DA, Grazziotin LR, Regier DA, Wordsworth S, Buchanan J, Phillips K, Ijzerman M. Addressing challenges of economic evaluation in precision medicine using dynamic simulation modeling. Value Health. 2020;23:566–73.
Patrinos G, CM-CP. Measuring the value of pharmacogenomics evidence. Wiley Online Library. 2017;102:739–741.
Payne K, Gavan SP, Wright SJ, Thompson AJ. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet. 2018;19:235–46.
Phillips KA, Ann Sakowski J, Trosman J, Douglas MP, Liang S-Y, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genet Med. 2014;16:251–7.
Berm EJJ, De Looff M, Wilffert B, Boersma C, Annemans L, Vegter S, Van Boven JFM, Postma MJ. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second update of the literature. PLoS ONE. 2016. https://doi.org/10.1371/JOURNAL.PONE.0146262.
Pliskin JS, Weinstein MC, Shepard DS. Utility functions for life years and health status. Oper Res. 1980;28:206–24.
Zeckhauser R, Shepard D. Where now for saving lives. Law Contemp Probl. 1976;40:5.
Pollard S, Weymann D, Chan B, et al. Defining a core data set for the economic evaluation of precision oncology. Value Health. 2022;25:1371–80.
Neumann PJ, Willke RJ, Garrison LP. A health economics approach to US value assessment frameworks—introduction: an ISPOR special task force report [1]. Value Health. 2018;21:119–23.
Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining Elements of value in health care—a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21:131–9.
Riley R, Lesteven D, Plun-Favreau J, Ferrara J, Kapitein P, Collins P. The value of knowing and knowing the value: improving the health technology assessment of complementary diagnostics. 2016.
Garrison L, Mestre-Ferrandiz J, Zamora B. The value of knowing and knowing the value: improving the health technology assessment of complementary diagnostics. 2016.
ICER 2020–2023 value assessment framework ICER.
Pitini E, De Vito C, Marzuillo C, D’andrea E, Rosso A, Federici A, Maria ED, Villari P. How is genetic testing evaluated? A systematic review of the literature. Eur J Hum Genet. 2018;26:605–15.
Goranitis I, Best S, Christodoulou J, Stark Z, Boughtwood T. The personal utility and uptake of genomic sequencing in pediatric and adult conditions: eliciting societal preferences with three discrete choice experiments. Genet Med. 2020;22:1311–9.
Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25:662–8.
NICE. Diagnostics assessment programme manual. 2011. https://www.nice.org.uk/media/default/about/what-we-do/nice-guidance/nice-diagnostics-guidance/diagnostics-assessment-programme-manual.pdf. Accessed 19 Nov 2023.
MSAC. Medical Services Advisory Committee process framework. 2016.
Cook JP, Golec J. How excluding some benefits from value assessment of new drugs impacts innovation. 2017. https://doi.org/10.1002/hec.3507.
Seo MK, Cairns J. How are we evaluating the cost-effectiveness of companion biomarkers for targeted cancer therapies? A systematic review. BMC Cancer. 2021. https://doi.org/10.1186/S12885-021-08725-4.
Te Aho o Te Kahu. He Pūrongo Mate Pukupuku o Aotearoa 2020: the state of cancer in New Zealand. 2020.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:1–7.
Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: scoping reviews. JBI Man Evid Synth. 2020. https://doi.org/10.46658/JBIMES-20-12.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Santos Gonzalez F, Mordaunt D, Stark Z, Dalziel K, Christodoulou J, Goranitis I. Microcosting diagnostic genomic sequencing: a systematic review. Genet Med. 2023;25: 100829.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18:2119–26.
Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil. 2011;92:2041–56.
Neumann PJ, Garrison LP, Willke RJ. The history and future of the ISPOR value flower: addressing limitations of conventional cost-effectiveness analysis. 2022. https://doi.org/10.1016/j.jval.2022.01.010.
Crummer E, Neumann PJ, Cohen JT, Kim DD. Use of novel and social elements of value in cost-effectiveness analysis. 2022. https://cevr.tuftsmedicalcenter.org/news/2022/use-of-novel-elements-of-value-in-cost-effectiveness-analyses. Accessed 19 Nov 2023.
Neumann PJ, Cohen JT, Hammitt JK, Concannon TW, Auerbach HR, Fang C, Kent DM. Willingness-to-pay for predictive tests with no immediate treatment implications: a survey of US residents. Health Econ. 2010;21:238–51.
Asch DA, Patton JP, Hershey JC. Knowing for the sake of knowing: the value of prognostic information. Med Decis Mak. 1990;10:47–57.
Best MC, Bartley N, Jacobs C, Juraskova I, Goldstein D, Newson AJ, Savard J, Meiser B, Ballinger M, Napier C. Patient perspectives on molecular tumor profiling: “Why wouldn’t you?” BMC Cancer. 2019;19:1–9.
Fifer S, Ordman R, Briggs L, Cowley A. Patient and clinician preferences for genetic and genomic testing in non-small cell lung cancer: a discrete choice experiment. J Pers Med. 2022. https://doi.org/10.3390/jpm12060879.
Weymann D, Veenstra DL, Jarvik GP, Regier DA. Patient preferences for massively parallel sequencing genetic testing of colorectal cancer risk: a discrete choice experiment. Eur J Hum Genet. 2018;26:1257–65.
Wong XY, Groothuis-Oudshoorn CG, Tan CS, van Til JA, Hartman M, Chong KJ, Ijzerman MJ, Wee H-L. Women’s preferences, willingness-to-pay, and predicted uptake for single-nucleotide polymorphism gene testing to guide personalized breast cancer screening strategies: a discrete choice experiment. Patient Prefer Adher. 2018;12:1837–52.
Sarki M, Ming C, Aissaoui S, et al. Intention to inform relatives, rates of cascade testing, and preference for patient-mediated communication in families concerned with hereditary breast and ovarian cancer and lynch syndrome: the Swiss CASCADE cohort. Cancers (Basel). 2022;14:1636.
Butow P, Davies G, Napier CE, et al. Assessment of the value of tumor variation profiling perceived by patients with cancer. JAMA Netw Open. 2020;3: e204721.
de Alava E, Pareja MJ, Carcedo D, Arrabal N, Garcia J-F, Bernabe-Caro R. Cost-effectiveness analysis of molecular diagnosis by next-generation sequencing versus sequential single testing in metastatic non-small cell lung cancer patients from a south Spanish hospital perspective. Expert Rev Pharmacoecon Outcomes Res. 2022;22:1033–42.
Hurry M, Eccleston A, Dyer M, Hoskins P. Canadian cost-effectiveness model of BRCA-driven surgical prevention of breast/ovarian cancers compared to treatment if cancer develops. Int J Technol Assess Health Care. 2020;36:104–12.
Butow P, Davies G, Napier CE, et al. Value of whole-genome sequencing to Australian cancer patients and their first-degree relatives participating in a genomic sequencing study. J Genet Couns. 2022;31:96–108.
Blouin-Bougie J, Amara N, Bouchard K, Simard J, Dorval M. Disentangling the determinants of interest and willingness-to-pay for breast cancer susceptibility testing in the general population: a cross-sectional Web-based survey among women of Quebec (Canada). BMJ Open. 2018;8: e016662.
Regier DA, Veenstra DL, Basu A, Carlson JJ. Demand for precision medicine: a discrete-choice experiment and external validation study. Pharmacoeconomics. 2020;38:57–68.
Mayer M, Selig K, Tuttelmann F, Dinkel A, Gschwend JE, Herkommer K. Interest in, willingness-to-pay for and willingness-to-recommend genetic testing for prostate cancer among affected men after radical prostatectomy. Fam Cancer. 2019;18:221–30.
McMullen C, Holup J, Davis JV, Foley P, Jacob L, Cottrell E, Bui DP, Wilfond B, Goddard KA. Discordant patient and clinician perspectives on the potential value of genetic services in safety-net clinics. J Health Care Poor Underserv. 2020;31:1347–63.
McCarthy MC, De Abreu LR, McMillan LJ, Meshcheriakova E, Cao A, Gillam L. Finding out what matters in decision-making related to genomics and personalized medicine in pediatric oncology: developing attributes to include in a discrete choice experiment. Patient. 2020;13:347–61.
Chow-White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics: a survey of medical oncologists. Hum Resour Health. 2017;15:1–9.
Clasen K, Gani C, Schroeder C, Riess O, Zips D, Schoffski O, Clasen S. Patient views on genetics and functional imaging for precision medicine: a willingness-to-pay analysis. Per Med. 2022;19:103–12.
Guo F, Hirth JM, Fuchs EL, Cofie LE, Brown V, Kuo Y-F, Fernandez ME, Berenson AB. Knowledge, attitudes, willingness to pay, and patient preferences about genetic testing and subsequent risk management for cancer prevention. J Cancer Educ. 2022;37:362–9.
Sun L, Cui B, Wei X, Sadique Z, Yang L, Manchanda R, Legood R. Cost-effectiveness of genetic testing for all women diagnosed with breast cancer in China. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14071839.
Young M-A, Forrest LE, Rasmussen V-M, James P, Mitchell G, Sawyer SD, Reeve K, Hallowell N. Making sense of SNPs: women’s understanding and experiences of receiving a personalized profile of their breast cancer risks. J Genet Couns. 2018;27:702–8.
Lourencao M, Simoes Correa Galendi J, Galvao HDCR, et al. Cost-Effectiveness of BRCA 1/2 genetic test and preventive strategies: using real-world data from an upper-middle income country. Front Oncol. 2022;12:951310.
Chandler Y, Schechter CB, Jayasekera J, et al. Cost effectiveness of gene expression profile testing in community practice. J Clin Oncol. 2018;36:554–62.
Eccleston A, Bentley A, Dyer M, Strydom A, Vereecken W, George A, Rahman N. A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health. 2017;20:567–76.
Asphaug L, Melberg HO. The cost-effectiveness of multigene panel testing for hereditary breast and ovarian cancer in Norway. MDM Policy Pract. 2019;4:2381468318821103.
Majem M, Alvarez R, Ortega AL, Ruiz de Alda L, Gordo R, Garcia JF, Ivanova-Markova Y, Gonzalez-Dominguez A, San Cristobal RS, Rojo F. Cost-benefit analysis of ALK diagnosis vs. non-diagnosis in patients with advanced non-small cell lung cancer in Spain. Glob Region Health Technol Assess. 2022;9:82–90.
Snowsill T, Coelho H, Huxley N, Jones-Hughes T, Briscoe S, Frayling IM, Hyde C. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2017;21:1–238.
Pereira C, Areia M, Dinis-Ribeiro M. Cost-utility analysis of genetic polymorphism universal screening in colorectal cancer prevention by detection of high-risk individuals. Dig Liver Dis. 2019;51:1731–7.
Simons MJHG, Retel VP, Ramaekers BLT, et al. Early cost effectiveness of whole-genome sequencing as a clinical diagnostic test for patients with inoperable stage IIIB, C/IV non-squamous non-small-cell lung cancer. Pharmacoeconomics. 2021;39:1429–42.
Ibarrondo O, Alvarez-Lopez I, Freundlich F, Arrospide A, Galve-Calvo E, Gutierrez-Toribio M, Plazaola A, Mar J. Probabilistic cost-utility analysis and expected value of perfect information for the oncotype multigenic test: a discrete event simulation model. Gac Sanit. 2020;34:61–8.
Martínez del Prado P, Alvarez-López I, Domínguez-Fernández S, Plazaola A, Ibarrondo O, Galve-Calvo E, Ancizar-Lizarraga N, Gutierrez-Toribio M, Lahuerta-Martínez A, Mar J. Clinical and economic impact of the 21-gene recurrence score assay in adjuvant therapy decision making in patients with early-stage breast cancer: pooled analysis in 4 Basque Country university hospitals. ClinicoEcono Outcomes Res. 2018:189–199.
Wei X, Cai J, Sun H, Li N, Xu C, Zhang G, Sui Y, Zhuang J, Zheng B. Cost-effectiveness analysis of UGT1A1*6/*28 genotyping for preventing FOLFIRI-induced severe neutropenia in Chinese colorectal cancer patients. Pharmacogenomics. 2019;20:241–9.
Kunst N, Stout NK, O’Brien G, Christensen KD, McMahon PM, Wu AC, Diller LR, Yeh JM. Population-based newborn screening for germline TP53 variants: clinical benefits, cost-effectiveness, and value of further research. J Natl Cancer Inst. 2022;114:722–31.
Neumann PJ, Thorat T, Shi J, Saret CJ, Cohen JT. The changing face of the cost-utility literature, 1990–2012. Value Health. 2015;18:271–7.
Achieving equity | Ministry of Health NZ. https://www.health.govt.nz/about-ministry/what-we-do/achieving-equity. Accessed 7 Dec 2023.
Drug Abacus—Drug Pricing Lab. https://www.drugpricinglab.org/tools/drug-abacus/. Accessed 7 Dec 2023.
Public board meeting agenda and papers: January 2022 | Public board meetings | Board | Who we are | About | NICE.
Zhang M, Bao Y, Lang Y, Fu S, Kimber M, Levine M, Xie F. What is value in health and healthcare? A systematic literature review of value assessment frameworks. Value Health. 2022;25(2):302–17.
Anonychuk A, Beastall G, Shorter S, Kloss-Wolf R, Neumann P. A framework for assessing the value of laboratory diagnostics. Healthc Manage Forum. 2012:25(3):S4–11.
Blonda A, Denier Y, Huys I, Simoens S. How to value orphan drugs? A review of European value assessment frameworks. Front Pharmacol. 2021;12:631527.
Lancet RW-T. Visibility of research: FUTON bias. 2002. thelancet.com.
Bartley N, Best M, Jacobs C, et al. Cancer patients’ views and understanding of genome sequencing: a qualitative study. J Med Genet. 2020;57:671–6.
Fisher ER, Pratt R, Esch R, Kocher M, Wilson K, Lee W, Zierhut HA. The role of race and ethnicity in views toward and participation in genetic studies and precision medicine research in the United States: a systematic review of qualitative and quantitative studies. Mol Genet Genomic Med. 2020. https://doi.org/10.1002/MGG3.1099.
Lau-Min KS, Varughese LA, Nelson MN, Cambareri C, Reddy NJ, Oyer RA, Teitelbaum UR, Tuteja S. Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer. 2022. https://doi.org/10.1186/S12885-022-09171-6.
Augustovski F, Alfie V, Alcaraz A, García Martí S, Drummond MF, Pichon-Riviere A. A value framework for the assessment of diagnostic technologies: a proposal based on a targeted systematic review and a multistakeholder deliberative process in Latin America. Value Health. 2021;24:486–96.
Gongora-Salazar P, Rocks S, Fahr P, Health OR-A-V. The use of multicriteria decision analysis to support decision making in healthcare: an updated systematic literature review. Amsterdam: Elsevier; 2023.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The research was conducted during AM’s tenure of a Health Research Council of New Zealand Clinical Research Training Fellowship and so the research was supported (in part) by funding from the Health Research Council of New Zealand.
Conflict of interest
AM, FGS, MK and PL have no conflicts of interest that are relevant to the content of this article.
Ethics approval
The study is a literature review and hence does not require ethical approval.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Data availability
The datasets used and/or analysed during this scoping review are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
AM, MW and PL conceived and designed the analysis. AM and FGS collected the data. AM, FGS, MW and PL performed the analysis. AM, FGS, MW and PL wrote the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Minhinnick, A., Santos-Gonzalez, F., Wilson, M. et al. How is Value Defined in Molecular Testing in Cancer? A Scoping Review. Appl Health Econ Health Policy (2024). https://doi.org/10.1007/s40258-024-00901-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s40258-024-00901-4