Abstract
Objectives
The aim of this study was to review the current evaluation and funding processes for new drugs in different developed countries, to provide a comparative framework with detailed, homogeneous, and up-to-date information.
Methods
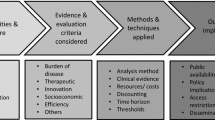
Scientific publications, reports and websites were reviewed between July and December 2021 using PubMed, Google Scholar, and grey literature sources. The main items searched were actors and processes, including timelines, characteristics of clinical and economic evaluations, participation of stakeholders, elements of price and reimbursement decisions, cost-effectiveness thresholds and specific funds. The analysed 13 countries were Australia, Canada, England, France, Germany, Italy, Japan, the Netherlands, Portugal, Scotland, South Korea, Spain and Sweden.
Results
Eight countries perform the assessment process separated from the pricing decision. Countries measure each drug’s added therapeutic value through multi-attribute value scales, algorithms, non-prescriptive lists of criteria, or quality-adjusted life years (QALYs). Health technology assessment (HTA) methodologies differ in their outcome measures, elicitation techniques, comparators, and perspectives. The criteria used for pricing and reimbursement include humanistic, clinical, and economic aspects. Only Scotland, England, the Netherlands, Canada and Portugal use explicit efficiency thresholds. Health care professionals participate in all assessment committees, and patients are becoming increasingly involved in most countries. The official time from marketing authorisation to the completion of the evaluation and pricing processes varied from 126 to 540 days.
Conclusions
Most analysed countries show a trend towards value-based approaches that consider value for money to society, but also other economic, clinical, and humanistic criteria. Good practices included robustness, transparency, independence, and participation.
Similar content being viewed by others
References
Morgan SG, Bathula HS, Moon S. Pricing of pharmaceuticals is becoming a major challenge for health systems. BMJ. 2020;13:368.
Auraaen A, Fujisawa R, Lagasnerie G de, Paris V. How OECD health systems define the range of good and services to be financed collectively [Internet]. Paris: OECD; 2016 [cited 2023 Apr 4]. https://www.oecd-ilibrary.org/social-issues-migration-health/how-oecd-health-systems-define-the-range-of-good-and-services-to-be-financed-collectively_5jlnb59ll80x-en.
Panteli D, Arickx F, Cleemput I, Dedet G, Eckhardt H, Fogarty E, et al. Pharmaceutical regulation in 15 European countries. Health Syst Transit. 2016;18(5):1–118.
Haute Autorité de Santé. Service médical rendu (SMR) Le médicament a-t-il suffisamment d’intérêt clinique pour être pris en charge par la solidarité nationale ? 2017.
Australian Government. Department of Health. Pharmaceutical Benefits Scheme (PBS) | Pharmaceutical Benefits Advisory Committee (PBAC) Membership [Internet]. 2021 [cited 2021 Oct 19]. https://www.pbs.gov.au/info/industry/listing/participants/pbac.
Hasegawa M, Komoto S, Shiroiwa T, Fukuda T. Formal implementation of cost-effectiveness evaluations in Japan: a unique health technology assessment system. Value Health. 2020;23(1):43–51.
SMC. Working with SMC. A Guide for Manufacturers [Internet]. 2020. https://www.scottishmedicines.org.uk/media/5498/working-with-smc-updated-october-2020.pdf.
Ophuis RH, Diemer FS. Specialist medicinal products assessment procedure. National Healthcare Institute [Internet]. 2020 [cited 2021 Sep 14]. https://english.zorginstituutnederland.nl/about-us/publications/reports/2020/05/11/specialist-medicinal-products-assessment-procedure.
MS Trust. Drug approval process [Internet]. 2020 [cited 2021 Oct 14]. https://mstrust.org.uk/a-z/drug-approval-process.
IQWiG. Drug approval and early benefit assessment in Germany [Internet]. [cited 2021 Sep 7]. https://www.iqwig.de/presse/im-fokus/neue-arzneimittel-zulassung-nutzenbewertung-erstattung/1-arzneimittel-zulassung-und-fruehe-nutzenbewertung-in-deutschland/.
Deutscher Bundestag. Finanzreform der gesetzlichen Krankenversicherung beschlossen [Internet]. Deutscher Bundestag. 2022 [cited 2023 Feb 28]. https://www.bundestag.de/dokumente/textarchiv/2022/kw42-de-gkv-finanzierungsstabilisierungsgesetz-916742.
HAS. Procédures d’évaluation anticipée « Fast - Tracking » par la Commission de la Transparence [Internet]. 2021. https://www.has-sante.fr/jcms/p_3082703/.
UK government. 150-day assessment for national applications for medicines [Internet]. GOV.UK. 2021 [cited 2021 Jul 19]. https://www.gov.uk/guidance/guidance-on-150-day-assessment-for-national-applications-for-medicines.
AIFA. Farmaci orfani [Internet]. 2021 [cited 2021 Oct 18]. https://aifa.gov.it/farmaci-orfani.
IQWIG. General Methods - Version 6.0 [Internet]. 2020. https://www.iqwig.de/methoden/general-methods_version-6-0.pdf.
Organisation for Economic Co-operation and Development (OECD). Pharmaceutical reimbursement and pricing in Germany [Internet]. 2018. https://www.oecd.org/els/health-systems/Pharmaceutical-Reimbursement-and-Pricing-in-Germany.pdf.
Vogler S. PPRI Pharma Brief: France [Internet]. 2020. https://jasmin.goeg.at/1686/1/PPRI_Pharma_Brief_FR_2020_final_Oct2020.pdf.
Agenzia Italiana del Farmaco. Farmaci innovativi [Internet]. 2022 [cited 2021 Jun 9]. https://aifa.gov.it/farmaci-innovativi.
AIFA. Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale. 2019; https://www.aifa.gov.it/documents/20142/1283800/Linee_guida_dossier_domanda_rimborsabilita.pdf.
AIFA. Criteri per la valutazione dell’innovativita’ [Internet]. 2017. https://www.aifa.gov.it/documents/20142/241044/Allegato_1_1.pdf/66558a13-543c-9643-e67b-85be01547465.
Raymakers AJN, Regier DA, Peacock SJ. Health-related quality of life in oncology drug reimbursement submissions in Canada: a review of submissions to the pan-Canadian Oncology Drug Review. Cancer. 2020;126(1):148–55.
NICE. Evidence. Guide to the methods of technology appraisal [Internet]. NICE; 2013 [cited 2021 Jul 28]. https://www.nice.org.uk/process/pmg9/chapter/evidence.
Core2Health. Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council [Internet]. 2019. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf.
SMC. Guidance to submitting companies for completion of New Product Assessment Form [Internet]. 2021. https://www.scottishmedicines.org.uk/media/6161/20210728-guidance-on-npaf.pdf.
NICE. NICE health technology evaluations: the manual [Internet]. 2022. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741.
NICE. Developing the scope. Guide to the methods of technology appraisal [Internet]. NICE; 2013 [cited 2021 Jul 28]. https://www.nice.org.uk/process/pmg9/chapter/developing-the-scope.
National Healthcare Institute. Assessment of established medical science and medical practice [Internet]. 2015 [cited 2021 Sep 15]. https://english.zorginstituutnederland.nl/about-us/publications/reports/2015/01/19/assessment-of-established-medical-science-and-medical-practice.
National Healthcare Institute. Criteria for the assessment of therapeutic value [Internet]. 2014 [cited 2021 Sep 15]. https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2014/07/01/criteria-voor-beoordeling-therapeutische-waarde.
Comissão de Avaliação de Tecnologías de Saúde. Metodología para Avaliação Farmacoterapéutica [Internet]. 2016. https://www.infarmed.pt/documents/15786/1963929/Metodologia%2bCATS/77f97467-01a9-4a82-8012-d6a608f420e1.
Australian Government. Department of Health. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee, Version 5.0 [Internet]. 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf.
Ministerio de Sanidad. Procedimiento normalizado de trabajo de evaluación clínica, evaluación económica y posicionamiento terapéutico para la redacción de Informes de Posicionamiento Terapéutico de medicamentos en el Sistema Nacional de Salud [Internet]. 2020. https://www.mscbs.gob.es/profesionales/farmacia/IPT/docs/20200708.PNT_elaboracion_IPT_CPF8Julio.pdf.
The Dental and Pharmaceutical Benefits Agency TLV. Health economic assessment of Strensiq in patients with hypophosphatasia [Internet]. 2021 [cited 2021 Sep 27]. https://www.tlv.se/download/18.87d7969177a1a94f16d4ad/1613498807170/bed210125_strensiq.pdf.
The Dental and Pharmaceutical Benefits Agency TLV. Health economic assessment of Zolgensma in spinal muscle atrophy [Internet]. 2021 [cited 2021 Sep 27]. https://www.tlv.se/download/18.2481e10b177db4d6d0ab7b0f/1615549930200/bed210224_zolgensma.pdf.
The Dental and Pharmaceutical Benefits Agency TLV. Health economics assessment of Sarclisa in multiple myeloma (isatuximab) [Internet]. 2021 [cited 2021 Sep 27]. https://www.tlv.se/download/18.77781fa17bb00c3f2233cb1/1630929350615/bes210826_sarclisa.pdf.
CADTH. Procedures for CADTH Reimbursement Reviews. 2021.
International Society For Pharmacoeconomics and Outcomes Research (ISPOR). Third Version of the Korean Economic Evaluation Guidelines [Internet]. 2020. https://www.ispor.org/publications/journals/value-outcomes-spotlight/vos-archives/issue/view/september-2020-supplement-spotlight-on-asia-pacific/third-version-of-the-korean-economic-evaluation-guidelines.
HAS. Choix méthodologiques pour l’évaluation économique à la HAS [Internet]. 2020 [cited 2021 Jun 25]. https://www.has-sante.fr/jcms/p_3197550/fr/guide-2020-choix-methodologiques-pour-l-evaluation-economique-a-la-has.
NICE. Incorporating economic evaluation [Internet]. NICE; 2020 [cited 2021 Jul 21]. https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation.
INFARMED. Avaliação terapêutica e económica [Internet]. 2021. https://www.infarmed.pt/web/infarmed/entidades/medicamentos-uso-humano/avaliacao-tecnologias-saude/avaliacao-terapeutica-e-economica.
Nederland Z. Farmaco-economie [Internet]. Zorginstituut Nederland; 2020 [cited 2022 Mar 13]. https://www.farmacotherapeutischkompas.nl/algemeen/farmaco-economie.
Heintz E, Arnberg K, Levin LÅ, Liliemark J, Davidson T. The impact of health economic evaluations in Sweden. Z Evid Fortbild Qual Gesundhwes. 2014;108(7):375–82.
NICE. The reference case [Internet]. NICE; 2020 [cited 2021 Jul 22]. https://www.nice.org.uk/process/pmg9/chapter/the-reference-case.
HAS. Notice de dépôt Modalités de dépôt d’un dossier d’évaluation économique auprès de la CEESP [Internet]. 2021. https://www.has-sante.fr/upload/docs/application/pdf/2013-08/notice_de_depot.pdf.
HAS. Choix méthodologiques pour l’analyse de l’impact budgétaire à la HAS [Internet]. 2016. https://www.has-sante.fr/upload/docs/application/pdf/2016-12/guide_methodologique__choix_methodologiques_pour_lanalyse_de_limpact_budgetaire_a_la_has_.pdf.
AIFA. Linee guida per la compilazione del Dossier a supporto della domanda di rimborsabilità e prezzo di un medicinale [Internet]. 2020. https://www.aifa.gov.it/documents/20142/0/AIFA_Linee+Guida_v.+16.9.2020+per+consultazione+pubblica.pdf/64f8d5b5-69df-a799-9ae7-36a5743d5f17.
Ortega Eslava A, Marín Gil R, Fraga Fuentes MD, López-Briz E, Puigventós Latorre F. Guía de evaluación económica e impacto presupuestario en los informes de evaluación de medicamentos [Internet]. SEFH. Sociedad Española de Farmacia Hospitalaria; 2016 [cited 2019 Mar 18]. https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf.
Lopert R, Viney R. Revolution then evolution: the advance of health economic evaluation in Australia. ZEFQ. 2014;108(7):360–6.
CADTH. Guidelines for the Economic Evaluation of Health Technologies: Canada (4th Edition). 2017.
Perelman J, Soares M, Mateus C, Duarte A, Faria R, Ferreira L, et al. Orientações Metodológicas para Estudos de Avaliação Económica [Internet]. INFARMED: Autoridade Nacional do Medicamento e Produtos de Saúde; 2019. https://www.infarmed.pt/documents/15786/4001413/Orienta%C3%A7%C3%B5es+metodol%C3%B3gicas+para+estudos+de+avalia%C3%A7%C3%A3o+econ%C3%B3mica+de+tecnologias+de+sa%C3%BAde/736f57a0-fa36-5e3e-65ae-00730de4dac9.
National Healthcare Institute. Guideline for economic evaluations in healthcare [Internet]. 2016 [cited 2021 Sep 16]. https://english.zorginstituutnederland.nl/about-us/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare.
Bae S, Lee S, Bae EY, Jang S. Korean guidelines for pharmacoeconomic evaluation (second and updated version): consensus and compromise. Pharmacoeconomics. 2013;31(4):257–67.
BOE. Real Decreto Legislativo 1/2015, de 24 de julio, por el que se aprueba el texto refundido de la Ley de garantías y uso racional de los medicamentos y productos sanitarios. [Internet]. 2015. https://www.boe.es/buscar/doc.php?id=BOE-A-2015-8343.
The Patented Medicine Prices Review Board. PMPRB Guidelines [Internet]. 2021 [cited 2021 Aug 17]. https://www.canada.ca/content/dam/pmprb-cepmb/documents/legislation/guidelines/PMPRB-Guidelines-en.pdf.
Department of Health and Social Care. The 2019 voluntary scheme for branded medicines pricing and access: chapters and glossary [Internet]. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/761834/voluntary-scheme-for-branded-medicines-pricing-and-access-chapters-and-glossary.pdf.
Ben Collins. Access to new medicines in the English NHS [Internet]. The King’s Fund. 2020 [cited 2021 Jul 21]. https://www.kingsfund.org.uk/publications/access-new-medicines-english-nhs.
Toumi M, Motrunich A, Millier A, Rémuzat C, Chouaid C, Falissard B, et al. Analysis of health economics assessment reports for pharmaceuticals in France—understanding the underlying philosophy of CEESP assessment. J Mark Access Health Policy. 2017;5(1):1344088.
HAS. Analyse d’impact budgétaire : la HAS enrichit l’évaluation médico-économique des produits de santé [Internet]. Haute Autorité de Santé. 2017 [cited 2021 Oct 18]. Available from: https://www.has-sante.fr/jcms/c_2747893/fr/analyse-d-impact-budgetaire-la-has-enrichit-l-evaluation-medico-economique-des-produits-de-sante
HAS. Commission de la Transparence [Internet]. 2020 [cited 2021 Jun 21]. https://www.has-sante.fr/jcms/c_412210/fr/commission-de-la-transparence.
GLOBALHealthPR. France.Market Access Pharma [Internet]. 2020 [cited 2021 Jun 21]. https://www.globalhealthpr.com/services/france/.
Ministerio de Salud. Boletín Oficial. Criterios para la negociación de los precios de los medicamentos reembolsados por el Servicio Nacional de Salud [Internet]. 2020 [cited 2021 Jun 9]. Available from: https://www.gazzettaufficiale.it/eli/id/2020/07/24/20A03810/sg
Yumoto T. Update of Drug Pricing System in Japan. 2018;
Ministério da Saúde de Portugal. Decreto-Lei n.o 97/2015. Diário da República n.o 105/2015 [Internet]. Jun 1, 2015. https://dre.pt/home/-/dre/67356991/details/maximized?p_auth=2jnk7Nkz.
INFARMED. Revisões de preços [Internet]. 2021. https://www.infarmed.pt/web/infarmed/entidades/medicamentos-uso-humano/avaliacao-economica/regulamentacao-preco-medicamentos/revisao_precos.
República Portuguesa Saúde, Serviço Nacional de Saúde, INFARMED. Methodological guidelines for economic evaluation studies of health technologies [Internet]. 2019 [cited 2021 Sep 6]. Available from: https://www.infarmed.pt/documents/15786/4001413/Orienta%C3%A7%C3%B5es+metodol%C3%B3gicas+para+estudos+de+avalia%C3%A7%C3%A3o+econ%C3%B3mica+de+tecnologias+de+sa%C3%BAde+%28EN%29/ebcfd930-94e2-c7e1-100a-ee1df3d76882
SMC. SMC modifiers used in appraising new medicines [Internet]. 2012. https://www.scottishmedicines.org.uk/media/3565/modifiers.pdf.
SMC. New Product Assessment Form [Internet]. 2020. https://www.scottishmedicines.org.uk/media/6159/20210728-new-product-assessment-form-npaf.docx.
Liu G, Wu EQ, Ahn J, Kamae I, Xie J, Yang H. The development of health technology assessment in asia: current status and future trends. Value Health Reg Issues. 2020;21:39–44.
Jang S, Byun J hye, Song I, Cho H. PPRI Pharma Profile South Korea 2018. Pharmaceutical Pricing and Reimbursement Information (PPRI), College of Pharmacy, Gachon University, National Health Insurance Service [Internet]. 2019. https://ppri.goeg.at/sites/ppri.goeg.at/files/inline-files/PPRI_Pharma_Profile_SouthKorea_final.pdf.
Pontén J, Rönnholm G, Skiöld P. Pharma Profile Sweden. Pharmaceutical Pricing and Reimbursement Information [Internet]. 2017 [cited 2021 Jun 10]. https://ppri.goeg.at/sites/ppri.goeg.at/files/inline-files/PPRI_Pharma_Profile_Sweden_2017_5.pdf.
National Healthcare Institute. Package advice in practice. Deliberations for arriving at a fair package [Internet]. 2018 [cited 2021 Sep 15]. https://english.zorginstituutnederland.nl/about-us/publications/reports/2018/09/05/package-advice-in-practice---deliberations-for-arriving-at-a-fair-package.
National Healthcare Institute. Budget impact analysis dossier template [Internet]. 2020 [cited 2021 Sep 15]. https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2020/07/22/format-budgetimpactanalyse.
Bae EY. Role of health technology assessment in drug policies: Korea. Value Health Reg Issues. 2019;18:24–9.
Core2Health. Precios de medicamentos y dispositivos médicos [Internet]. 2021 [cited 2021 Oct 29]. Available from: https://c2h.niph.go.jp/assessment/price-setting/
CADTH. Guidelines for the Economic Evaluation of Health Technologies: Canada (4th Edition). Appendix Worked Example. 2017;
National Healthcare Institute., Zwaap J, Knies S, van der Meijden C, Staal P, van der Heiden L. Cost-effectiveness in practice. [Internet]. 2015 [cited 2021 Sep 15]. https://english.zorginstituutnederland.nl/about-us/publications/reports/2015/06/16/cost-effectiveness-in-practice.
NICE. The appraisal of the evidence and structured decision-making | Guide to the methods of technology appraisal [Internet]. NICE; 2013 [cited 2023 Aug 10]. Available from: https://www.nice.org.uk/process/pmg9/chapter/the-appraisal-of-the-evidence-and-structured-decision-making#decision-making
Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–61.
Donovan P. Access to unregistered drugs in Australia. Aust Prescr. 2017;40(5):194–6.
Lybrand S, Wonder M. Analysis of PBAC submissions and outcomes for medicines (2010–2018). Int J Technol Assess Health Care. 2020;36(3):224–31.
Bae EY, Kim HJ, Lee HJ, Jang J, Lee SM, Jung Y, et al. Role of economic evidence in coverage decision-making in South Korea. PLoS ONE. 2018;13(10): e0206121.
Forest J. L’accès au marché des médicaments en France au travers des contrats de paiement à la performance: solution crédible et extrapolable? Sciencespharmaceutiques [Internet]. 2020. https://tel.archives-ouvertes.fr/UFR_PHARMAGRENOBLE/dumas-02470370v1.
Russo P, Zanuzzi M, Carletto A, Sammarco A, Romano F, Manca A. Role of Economic Evaluations on Pricing of Medicines Reimbursed by the Italian National Health Service. Pharmacoeconomics. 2023;41(1):107–17.
Svensson M, Nilsson FOL, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33(11):1229–36.
Balijepalli C, Gullapalli L, Druyts E, Yan K, Desai K, Barakat S, et al. Can standard health technology assessment approaches help guide the price of orphan drugs in Canada? A review of submissions to the Canadian agency for drugs and technologies in health common drug review. Clinicoecon Outcomes Res. 2020;24(12):445–57.
Kim H, Byrnes J, Goodall S. Health technology assessment in Australia: the pharmaceutical benefits advisory committee and medical services advisory committee. Value Health Re Issues. 2021;24:6–11.
SMC. Guide to the Scottish Medicines Consortium [Internet]. 2018. https://www.scottishmedicines.org.uk/media/3574/20180712-a-guide-to-the-scottish-medicines-consortium.pdf.
Patented Medicine Prices Review Board. Roles and Responsiblities of Board Members [Internet]. 2020 [cited 2022 Apr 21]. https://www.canada.ca/en/patented-medicine-prices-review/corporate/organizational-structure/roles-responsiblities-board-members.html.
HIRA - Health Insurance Review & Assessment Service - Korea. Drug Benefit Coverage Assessment Committee [Internet]. 2021. https://www.hira.or.kr/dummy.do?pgmid=HIRAA030051000006.
AIFA. Commissioni tecnico-consultive [Internet]. [cited 2021 Jun 9]. https://aifa.gov.it/commissioni-tecnico-consultive.
INFARMED. Comissão de Avaliação de Tecnologias de Saúde (CATS) [Internet]. 2021. https://www.infarmed.pt/web/infarmed/institucional/estrutura-e-organizacao/comissoes-tecnicas-especializadas/comissao-de-avaliacao-de-tecnologias-de-saude.
Ministerio da Saúde de Portugal. Despacho 5847/2016, de 2 de maio [Internet]. Diário da República No. 84 Série II May 2, 2016. https://dre.pt/application/conteudo/74313786.
Ministerio da Saúde de Portugal. Despacho n.o 7069/2016. Diário da República, 2.a série. N.o 103 [Internet]. May 30, 2016. https://dre.pt/application/conteudo/74557716.
Ministerio da Saúde de Portugal. Despacho n.o 1878/2017. Diário da República, 2.a série. N.o 46 [Internet]. Mar 6, 2017. https://dre.pt/application/conteudo/106550965.
National Institute for Health and Care Excellence. Guide to the processes of technology appraisal. NICE; 2018 [cited 2021 Jul 21]. https://www.nice.org.uk/process/pmg19/chapter/the-appraisal-process#evidence-review-group-report.
Kamae I, Thwaites R, Hamada A, Fernandez JL. Health technology assessment in Japan: a work in progress. J Med Econ. 2020;23(4):317–22.
National Healthcare Institute. Working committees and members of the committees from the Scientific Advisory Board (WAR). 2021 [cited 2021 Sep 15]. https://www.zorginstituutnederland.nl/over-ons/commissies/wetenschappelijke-adviesraad-war/werkcommissies-en-leden.
Gouvernment of Canada. Patented Medicine Prices Review Board. 2020 [cited 2021 Jul 29]. http://pmprb-cepmb.gc.ca.
INFARMED. Direção de Avaliação das Tecnologias de Saúde (DATS). 2021. https://www.infarmed.pt/web/infarmed/institucional/estrutura-e-organizacao/dats.
GBA. Recopilación de datos relacionados con la aplicación de nuevos medicamentos. 2020 [cited 2021 Sep 8]. https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/anwendungsbegleitende-datenerhebung/.
Ministerio de Sanidad. Plan para la consolidación de los Informes de Posicionamiento Terapéutico de los medicamentos en el Sistema Nacional de Salud. 2020. https://www.mscbs.gob.es/profesionales/farmacia/IPT/docs/20200708.Plan_de_accion_para_la_consolidacion_de_los_IPT.actCPF8Julio.pdf.
AIFA. Reglamento de organización y funcionamiento del Comité Consultivo Técnico y Científico y del Comité de Precios y Reembolsos. 2014.
Patient and public involvement policy. Public Involvement Programme. Public involvement. NICE and the public. NICE Communities. NICE; 2021 [cited 2021 Sep 10]. https://www.nice.org.uk/about/nice-communities/nice-and-the-public/public-involvement/public-involvement-programme/patient-public-involvement-policy.
Mesana L, Syed IA, Constantin J, Tardivel C, Wang X, Pruce D, et al. Patient involvement in health technology assessments: an international comparison. Value Health. 2018;21:S54.
HAS. Comprendre l’évaluation des médicaments. Haute Autorité de Santé. 2019 [cited 2021 Jun 24]. https://www.has-sante.fr/jcms/c_412115/fr/comprendre-l-evaluation-des-medicaments.
Oliveira M. Projeto INCLUIR: Patient involvement in health tecnhnology assessment in Portugal. 2019. https://cddf.org/wp-content/uploads/2019/07/Day1_1630_Margarida-Oliveira.pdf.
HAS. Commission d’évaluation économique et de santé publique. Haute Autorité de Santé. 2018 [cited 2021 Jun 21]. https://www.has-sante.fr/jcms/c_419565/fr/commission-d-evaluation-economique-et-de-sante-publique.
HAS. Règlement intérieur de la commission d’évaluation économique et de santé publique. 2020. Available from: https://www.has-sante.fr/upload/docs/application/pdf/2018-10/reglement_interieur_ceesp.pdf
France Assos Santé. Qui sommes-nous ? France Assos Santé. 2019 [cited 2021 Jun 21]. https://www.france-assos-sante.org/presentation/qui-sommes-nous/.
G-BA. AMNOG - Evaluación de beneficios de medicamentos de acuerdo con § 35a SGB V - Comité Conjunto Federal. 2011 [cited 2021 Sep 7]. https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/nutzenbewertung-35a/.
NICE. Introduction. Guide to the processes of technology appraisal. NICE; 2018 [cited 2021 Jul 23]. https://www.nice.org.uk/process/pmg19/chapter/introduction
Kloeke K van L, Artz L. Pricing & Reimbursement Laws and Regulations 2021. Netherlands. Global Legal Insights. 2021 [cited 2021 Sep 14]. https://www.globallegalinsights.com/practice-areas/pricing-and-reimbursement-laws-and-regulations/netherlands.
National Healthcare Institute. Assessment of outpatient medicines for the benefit of the Medicine Reimbursement System (GVS). 2021 [cited 2021 Sep 14]. https://english.zorginstituutnederland.nl/about-us/tasks-of-the-national-health-care-institute/assessment-of-outpatient-medicines-for-the-benefit-of-the-medicine-reimbursement-system-gvs.
Ministerio de Sanidad y Consumo. BOE-A-2002-24821 Instrucción de 13 de diciembre de 2002, de la Subsecretaría, por la que se coordinan los procedimientos administrativos relativos a autorización de comercialización y a financiación con fondos públicos de las especialidades farmacéuticas de uso humano. 2002. https://www.boe.es/buscar/doc.php?id=BOE-A-2002-24821.
Autoridad Independiente de Responsabilidad Fiscal. Gasto hospitalario del sistema nacional de salud: farmacia e inversión en bienes de equipo. 2019. https://www.airef.es/wp-content/uploads/2020/10/SANIDAD/ANEXOS/Documento-Anexo-7.-Farmacia-Hospitalaria.pdf.
Ministerio de Sanidad, Consumo y Bienestar. Reglamento interno de la Comisión Interministerial de Precios de Medicamentos y Productos sanitarios (CIMP). 2019.
Journal officiel de la République française. Artículo D162-2-5. Code de la sécurité sociale. 2018. https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000032092789?etatTexte=VIGUEUR&etatTexte=VIGUEUR_DIFF#LEGIARTI000006735445.
Journal officiel de la République française. Article L162-17-3. Code de la sécurité sociale. 2016. https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000031931783.
GKV-Spitzenverband. Wir über uns - GKV-Spitzenverband. GKV-Spitzenverband; 2020 [cited 2021 Sep 7]. https://www.gkv-spitzenverband.de/gkv_spitzenverband/der_verband/wir_ueber_uns.jsp.
NICE. Cancer Drugs Fund. NICE. NICE; 2021 [cited 2021 Jul 21]. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/cancer-drugs-fund.
NHS Scotland. Ultra-orphan medicines. National Services Scotland. 2021 [cited 2021 Aug 5]. https://www.nss.nhs.scot/specialist-healthcare/financial-risk-share/ultra-orphan-medicines/.
Ministerie van Volksgezondheid W en S. Conditional inclusion of orphan drugs, conditionals and exceptionals in basic health care - About us - National Health Care Institute. Ministerie van Volksgezondheid, Welzijn en Sport; 2020 [cited 2023 Mar 14]. https://english.zorginstituutnederland.nl/about-us/tasks-of-the-national-health-care-institute/conditional-inclusion-of-orphan-drugs-conditionals-and-exceptionals.
NHS England. NHS England announces new Innovative Medicines Fund to fast-track promising new drugs. 2021 [cited 2021 Dec 22]. https://www.england.nhs.uk/2021/07/nhs-england-announces-new-innovative-medicines-fund-to-fast-track-promising-new-drugs/.
Scottish Goverment. New Medicines Fund and Rare Conditions Medicines Fund information: FOI release. 2022 [cited 2022 Apr 26]. http://www.gov.scot/publications/foi-202200287608/.
NHS Scotland. Inherited metabolic disorders. National Services Scotland. 2021 [cited 2021 Aug 5]. https://www.nss.nhs.scot/specialist-healthcare/financial-risk-share/inherited-metabolic-disorders/.
AIFA. Elenchi farmaci innovativi ai sensi dell’art. 1, commi 402, 403 e 404, della legge 11/12/2016, n. 232 (Legge di Bilancio 2017). 2021. https://www.aifa.gov.it/documents/20142/1444653/1_Elenchi_farmaci_innovativi_fondi_Legge_Bilancio2017_28.05.2021.pdf.
National Healthcare Institute. Conditional inclusion of orphan drugs, conditionals and exceptionals in basic health care. 2020 [cited 2021 Sep 20]. https://english.zorginstituutnederland.nl/about-us/tasks-of-the-national-health-care-institute/conditional-inclusion-of-orphan-drugs-conditionals-and-exceptionals.
Barnieh L, Manns B, Harris A, Blom M, Donaldson C, Klarenbach S, et al. A synthesis of drug reimbursement decision-making processes in organisation for economic co-operation and development countries. Value Health. 2014;17(1):98–108.
Hasan SS, Kow CS, Dawoud D, Mohamed O, Baines D, Babar ZUD. Pharmaceutical policy reforms to regulate drug prices in the Asia Pacific Region: the Case of Australia, China, India, Malaysia, New Zealand, and South Korea. Value Health Reg Issues. 2019;18:18–23.
Beletsi A, Koutrafouri V, Karampli E, Pavi E. Comparing use of health technology assessment in pharmaceutical policy among earlier and more recent adopters in the European Union. Value in Health Regional Issues. 2018;1(16):81–91.
Angelis A, Lange A, Kanavos P. Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. Eur J Health Econ. 2018;19(1):123–52.
Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1):1447828.
Yanovskiy M, Levy ON, Shaki YY, Zigdon A, Socol Y. Cost-effectiveness threshold for healthcare: justification and quantification. Inquiry. 2022;13(59):00469580221081438.
Vallejo-Torres, L C, García-Lorenzo, B, Castilla, I. Valor Monetario de un Año de Vida Ajustado por Calidad: Estimación empírica del coste de oportunidad en el Sistema Nacional de Salud. 2015. (Ministerio de Sanidad, Servicios Sociales e Igualdad. Servicio de Evaluación del Servicio Canario de la Salud; Informes de Evaluación de Tecnologías Sanitarias).
Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28.
Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–30.
Harris AH, Hill SR, Chin G, Li JJ, Walkom E. The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994–2004. Med Decis Making. 2008;28(5):713–22.
Matusewicz W, Godman B, Pedersen HB, Fürst J, Gulbinovič J, Mack A, et al. Improving the managed introduction of new medicines: sharing experiences to aid authorities across Europe. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):755–8.
Epstein D, Espín J. Evaluation of new medicines in Spain and comparison with other European countries. Gac Sanit. 2020;34(2):133–40.
Drummond MF, Schwartz JS, Jönsson B, Luce BR, Neumann PJ, Siebert U, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24(3):244–58.
Zamora B, Maignen F, O’Neill P, Mestre-Ferrandiz J, Garau M. Comparing access to orphan medicinal products in Europe. Orphanet J Rare Dis. 2019;14(1):95.
Iglesias-López C, Agustí A, Vallano A, Obach M. Financing and reimbursement of approved advanced therapies in several european countries. Value Health. 2023;26(6):841–53.
European Commission. Directive 2004/109/EC of the European Parliament and of the Council of 15 December 2004 on the harmonisation of transparency requirements in relation to information about issuers whose securities are admitted to trading on a regulated market and amending Directive 2001/34/EC. OJ L Dec 15, 2004. http://data.europa.eu/eli/dir/2004/109/oj/eng
Newton M, Stoddart K, Travaglio M, Troein P. EFPIA Patients W.A.I.T. Indicator 2022 Survey. 2023. https://www.efpia.eu/media/s4qf1eqo/efpia_patient_wait_indicator_final_report.pdf.
Post C, Van Laarhoven HWM, Hollak C. Time to access to novel anticancer drugs in Europe, a case study in seven European countries. JCO. 2022;40(16 suppl):1586–1586.
Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending Directive 2011/24/EU. 2021 [cited 2023 Feb 27]. http://www.bloomsburycollections.com/book/fundamental-texts-on-european-private-law-1.
Franken M, le Polain M, Cleemput I, Koopmanschap M. Similarities and differences between five european drug reimbursement systems. Int J Technol Assess Health Care. 2012;28(4):349–57.
Verghese NR, Barrenetxea J, Bhargava Y, Agrawal S, Finkelstein EA. Government pharmaceutical pricing strategies in the Asia-Pacific region: an overview. J Mark Access Health Policy. 2019;7(1):1601060.
Sorenson C, Drummond M, Kanavos P. Ensuring value for money in health care: the role of health technology assessment in the European Union. {S.l.]: European Observatory on Health Systems and Policies; 2008. 156 p.
Rejon-Parrilla JC, Espin J, Epstein D. How innovation can be defined, evaluated and rewarded in health technology assessment. Heal Econ Rev. 2022;12(1):1.
Zozaya, N, Villaseca J, Abdalla F, Fernández I, Hildago-Vega A. The assessment and funding processes of drugs in Spain and other OECD countries: Where are we and where are we going? Madrid: Fundación Weber; 2023 [cited 2023 Mar 9]. https://weber.org.es/publicacion/the-assessment-and-funding-processes-of-drugs-in-spain-and-other-oecd-countries-where-are-we-and-where-are-we-going/.
Bae EY, Hong J, Bae S, Hahn S, An H, Hwang EJ, et al. Korean guidelines for pharmacoeconomic evaluations: updates in the third version. Appl Health Econ Health Policy. 2022;20(4):467–77.
The Dental and Pharmaceutical Benefits Agency TLV. General guidelines for economic evaluations from the Pharmaceutical Benefits Board. 2003 [cited 2021 Sep 27]. https://www.tlv.se/download/18.2e53241415e842ce95514e9/1510316396792/Guidelines-for-economic-evaluations-LFNAR-2003-2.pdf.
Sacristán JA, Oliva J, Campillo-Artero C, Puig-Junoy J, Pinto-Prades JL, Dilla T, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? Gac Sanit. 2020;34(2):189–93.
European Central Bank. Euro foreign exchange reference rates. European Central Bank. 2023 [cited 2023 Dec 11]. https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/index.en.html.
Funding
No funding was received for this study. However, it should be mentioned that AbbVie sponsored an activity during the initial discussion of this manuscript. This manuscript was developed independently of AbbVie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have no financial or proprietary interests in any material discussed in this article. The authors declare that they have no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication (from patients/participants)
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors' Contributions
AHV, MAC, PGP, JMF, JO, JLT design the conception of the work; NZ, JV, FA and IF reviewed the literature and interpreted the data; NZ, JV and FA drafted the work; IF, BCN, MAC, PGP, JMF, JO, JLT and AHV revised it critically for important intellectual content.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zozaya, N., Villaseca, J., Fernández, I. et al. A Review of Current Approaches to Evaluating and Reimbursing New Medicines in a Subset of OECD Countries. Appl Health Econ Health Policy 22, 297–313 (2024). https://doi.org/10.1007/s40258-023-00867-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-023-00867-9