Abstract
Background
The total economic burden of cancer reflects direct and indirect costs, including productivity loss due to employment change, absenteeism, and presenteeism of patients and caregivers.
Objective
This study estimated the magnitude of employment decrease, work absence (WA), short-term disability (STD), long-term disability (LTD), and associated indirect costs among employees newly diagnosed with metastatic versus non-metastatic cancer in the USA.
Methods
IBM® MarketScan® Commercial Claims and Encounters and Health and Productivity Management databases were used to identify employees aged 18–64 years and newly diagnosed with any cancer from 2009 to 2019. Proportions of patients with employment decrease, WA, STD, and LTD claims, and number of days missing from work were summarized by metastatic status during the first 12 months after diagnosis and the entire follow-up period. Subgroup analyses were conducted by age (< 50 years, ≥ 50 years) and cancer type (breast, lung, colon, pancreatic, and liver cancer).
Results
During the first year after diagnosis, compared to patients without metastases, significantly higher proportions of patients with metastases had employment decrease and STD or LTD claims (p < 0.001). The mean total number of days missing from work for patients with versus without metastases was 33.39 versus 14.91 (ratio = 2.40), 64.05 versus 27.15 (ratio = 2.36), and 105.93 versus 46.29 (ratio = 2.29) days within 3, 6, and 12 months after diagnosis, respectively. Estimates of indirect cost differences between the two groups ranged from $6,877 to $22,283 in the first year.
Conclusion
Earlier detection of cancer may reduce productivity loss of patients and indirect costs by initiating treatment before cancer progresses to late stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Productivity loss is an important component of overall cancer burden. |
Patients newly diagnosed with late-stage cancers had significantly higher productivity loss compared to early-stage cancers. |
1 Introduction
Cancer is a leading cause of death in the United States (USA), where more than half a million people died from cancer in 2019 [1]. Earlier detection, usually when cancer is at an early stage and still localized prior to metastasis, is essential to improve survival and reduce cost. Approximately 89% of patients diagnosed with localized cancer survive 5 years, in contrast to only 21% of patients diagnosed with metastatic cancer [2]. For lung cancer, which was the primary cause of cancer death in the USA in 2019 [3], the 5-year survival rates of early and late diagnosis are 56% versus 5%, respectively [4]. The 5-year mortality rates for stage 1 versus stage 4 are 2% versus 73% for breast cancer; 6% versus 87% for colorectal cancer; 10% versus 78% for ovarian cancer; and 11% versus 45% for head and neck cancer, as calculated using data from the Surveillance, Epidemiology, and End Results (SEER) database [5].
Cancers diagnosed at later stages are associated with elevated direct medical costs. In a recent study, the cost of healthcare in the year after a cancer diagnosis was significantly higher among patients diagnosed at later versus earlier cancer stages, ranging from 1.9 to 4.7 times higher for colorectal and ovarian cancer, respectively, amounting to as much as a $133,000 difference in mean annual costs of care [6]. It is estimated that $26 billion could be saved from early cancer diagnosis in annual treatment cost alone in the USA [7].
In addition to direct medical costs, cancer and associated treatments have other detrimental economic effects for both patients and caregivers [8], including negative impacts on employment status change, absenteeism, and presenteeism, which are also associated with indirect costs to employers and contribute to the total costs of cancer to the society [9]. Secondary analysis of clinical trial patients found that metastatic disease was associated with a change in employment status and level such as quitting jobs or converting from full-time to part-time jobs [10]. One retrospective cohort study among cancer patients with seven types of cancer and matched non-cancer controls found a monthly difference of $945 in indirect costs due to absent workdays and short-term disability days (2.0 and 5.0 days, respectively) for cancer patients compared to non-cancer patients [11]. In addition, a systematic review indicated that much of the evidence on the indirect costs of cancer compares outcomes by treatment, does not consider the impact of early- versus late-stage diagnosis, focuses narrowly on specific cancer types (most commonly breast cancer in women), and uses a survey research study design, which may be associated with recall bias if the recall period is long [12]. The difference in indirect costs by cancer stage provides valuable information to both employers and policy makers regarding the value of early cancer detection and/or cancer treatments.
The primary objective of the present study was to estimate the productivity loss in terms of employment decrease and work loss [work absence (WA), short-term disability (STD), and long-term disability (LTD)] and associated indirect costs among patients newly diagnosed with cancer at early versus late stages.
2 Methods
2.1 Study Design and Data Source
This was an observational, retrospective cohort study of de-identified US healthcare claims data spanning from 30 June 2008 to 30 June 2020 from the IBM® MarketScan® Commercial Claims and Encounters database and to 19 December 2019 from the IBM® MarketScan® Health Productivity and Management database (HPM).
The Commercial database contains the inpatient and outpatient encounters, and outpatient drug prescription information of employees (and their dependents) from over 300 employers, covered under a variety of fee-for-service and managed-care health plans, including 24.8 million lives in 2019, across all geographic regions of the USA [13]. Demographic information is available as well as information derived from administrative claims regarding healthcare services and related diagnoses. Individuals can be followed longitudinally for as long as they are with the data contributor, meaning they can be followed while with an employer regardless of changes in health plan and enrollment can be verified for this period.
The HPM database contains WA, STD, and LTD data from a subset of employees contributing to the Commercial database, including 3.7 million lives in 2019, fully linkable to the corresponding medical and pharmacy claims data for these employees.
All patient records were de-identified and fully compliant with the US patient confidentiality requirements (the Health Insurance Portability and Accountability Act, 1996). Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
2.2 Patient Selection
Patients with at least one inpatient cancer claim or two non-diagnostic outpatient cancer claims at least 30 days apart were selected between 1 January 2009 and 30 June 2020 for the employment cohort and between 1 January 2009 and 31 December 2019 for work loss cohorts. The requirement for a second outpatient cancer claim was used to exclude cases related to evaluation of a potential disease (later ruled negative) in addition to minimize possible coding errors. Relevant claims were identified using diagnosis codes from the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM). The date of the earliest cancer diagnosis was defined as the index date. Patients included in the employment and work loss cohorts were required to (1) be between 18 and 64 years old on the index date, (2) have at least 6 months of continuous enrollment with medical and pharmacy benefits before (pre-index period) and 1 month after (post-index period) the index date, and (3) have no evidence of cancer diagnosis during the pre-index period, >1 primary cancer diagnosis on index date, or pregnancy/childbirth during the study period. Patients eligible for the work loss cohorts were further identified in the HPM database with at least 1 month of eligibility before and after the index date for WA, STD, or LTD; eligibility in this case means that the information for the relevant work loss type was available, regardless of whether the patient had a claim for WA, STD, or LTD.
Upon identifying eligible patients for each cohort (employment, WA, STD, LTD), newly diagnosed patients with cancer were further stratified into early (without metastasis) versus late (with metastasis) stage. Patients with a diagnosis for secondary malignant neoplasm on or within 30 days following the index date were categorized to the metastatic cohort and the remaining patients were categorized to the non-metastatic cohort.
The full post-index period was variable in length (minimum 1 month) starting on the index date and ending with the earliest of (1) disenrollment for employment cohort or end of WA, STD, or LTD eligibility for work loss cohorts, (2) end of study period (30 June 2020 for employment cohort and 31 December 2019 for work loss cohorts), or (3) a claim for metastasis diagnosis among patients in the non-metastatic cohort (Figs. 1, 2).
2.3 Outcomes
2.3.1 Patient Characteristics
Patient age, sex, and cancer type were measured on the index date. The National Cancer Institute (NCI) modified form of the Charlson Comorbidity Index (the NCI Comorbidity Index) were measured during the pre-index period.
2.3.2 Employment Status
Among patients whose employment status indicated Active Full Time or Part Time in the month prior to the index date, the occurrence of an employment decrease was defined as changing from Active Full Time to either Part Time or Quit Job, or from Part Time to Quit Job, measured during the full post-index period and during months 1–3, months 4–6, and months 7–12 after the index date. The cumulative proportions of patients who had their first employment status decrease during corresponding time periods were summarized.
2.3.3 Work Loss
Outcomes for the work-loss cohorts (WA, STD, and LTD) include proportions of patients with at least 1 day of the respective category of work loss and number of days lost per-patient per-month (PPPM) among the patients who had at least 1 day of corresponding work loss during the full post-index period and during months 1–3, months 4–6, and months 7–12 after the index date. Indirect costs associated with work loss included the costs paid to employee, calculated by multiplying the number of days lost with an age-, sex-, and region-specific estimated daily wage rate. We assumed that employers typically pay 100% of wages for WA and 70% for STD or LTD benefit [14]. Wages were calculated using the national average wage rates from the US Bureau of Labor Statistics from the year 2020 [15].
2.3.4 Statistical Analysis
Employment and work-loss outcomes were reported for eligible patients during the full post-period and during months 1–3, months 4–6, and months 7–12 after cancer diagnosis and compared between metastatic versus non-metastatic cohorts. Only patients who had information about employment, WA, STD, or LTD during corresponding time periods were included in the analyses. Mean, standard deviation (SD), and median were reported for continuous variables. Frequencies and percentages were reported for categorical variables. Statistical tests of significance for differences between cancer patients with versus without metastasis were conducted. Chi-square tests were used to evaluate the statistical significance of differences for categorical variables; t tests were used for continuous variables. The alpha level for all statistical tests was 0.05. Ratios of mean number of days missing from work aggregating WA, STD, and LTD were calculated between metastatic versus non-metastatic cohorts. All data analyses were conducted using WPS version 4.1 (World Programming, UK).
2.3.5 Subgroup Analyses
Work-loss outcomes for metastatic and non-metastatic patients were also summarized by age (younger than 50 years versus 50 years and older) and for several cancer types (breast, lung, colon, pancreatic, and liver cancers).
2.3.6 Sensitivity Analyses
To account for the heterogeneity in wage rates across different industries, sensitivity analyses were conducted to estimate indirect costs based on wage rates in high-wage industries and low-wage industries. Data from the Bureau of Labor Statistics [16] showed the mean weekly wages of the three lowest-wage industries (leisure and hospitality, retail trade, and other services) as being 64% of the overall mean wage, while the mean weekly wages of the three highest-wage industries (mining and logging, information, and utilities) were 162% of the overall mean wage. Furthermore, calculating indirect costs based on wages only may underestimate the true financial impact to employers, depending on how easy it is to replace an absent worker, the degree to which a worker functions in an integrated team, or the time sensitivity of the worker’s output. Across all industries, Nicholson et al. [17] suggested that a median wage multiplier of 1.28 provides a more accurate measure of full indirect costs to employers estimated across 35 different jobs (i.e., the cost to employers is 28% higher than the worker’s wage). Therefore, we also calculated the indirect costs by applying this multiplier as sensitivity analyses.
3 Results
3.1 Patient Characteristics
Numbers of eligible patients varied by study cohorts: 1,226,830 for employment cohort, 30,785 for WA cohort, 171,119 for STD cohort, and 158,328 for LTD cohort (Fig. 2). The smaller patient counts for WA, STD, and LTD cohorts were reflective of the HPM database being a subset of the Commercial database. A minority (< 10%) of patients had metastases at cancer diagnoses: 9.2% for the employment cohort, 7.9% for the WA cohort, 8.4% for the STD cohort, and 8.4% for the LTD cohort.
The patients in the employment cohort were on average aged 54 years and the patients in the WA and other work loss cohorts were on average aged 53 years. About half of the patients in the employment cohort were female (metastatic 55.2% and non-metastatic 51.5%), whereas most patients in the WA cohort were male (metastatic 68.4% and non-metastatic 73.2%). Given the similarities between WA and other work loss cohorts, baseline characteristics for the WA cohort are presented in Table 1, whereas those for the STD and LTD cohorts can be found in Table S1 in the Online Supplemental Material (OSM).
Duration of follow-up was shorter for metastatic versus non-metastatic patients in all cohorts: employment (mean [SD]: 20.9 [22.1] months versus 31.7 [27.6] months, p < 0.001) and WA (mean [SD]: 30.1 [30.1] months versus 50.0 [34.6] months, p < 0.001), possibly influenced by the differences in survival and employment status between metastatic versus non-metastatic patients. For both the employment and WA cohorts, the majority of patients ended follow-up due to end of enrollment/eligibility, with < 10% of non-metastatic patients censored for a claim for metastasis diagnosis.
The mean NCI Comorbidity Index was higher among metastatic versus non-metastatic patients for both employment (0.37 vs. 0.30, p < 0.001) and WA (0.30 vs. 0.24, p < 0.001) patients, with the most common comorbidity conditions being mild-to-moderate diabetes and chronic pulmonary disease. The most common cancer types included breast, lung/bronchus, and colon/rectum (Table 1).
3.2 Employment Status
Employment status change since cancer diagnosis was assessed for 53% of metastatic and 55% of non-metastatic patients with Active Full Time (98%) or Active Part Time (2%) status in the month prior to the index date. During all the time periods assessed, a higher proportion of patients with metastasis had employment decrease versus patients without metastases (months 1–3: 13% vs. 6%, months 4–6: 17% vs. 8%, months 7–12: 22% vs. 11% (p < 0.001)). During the full post-index period, the proportion of patients with an employment decrease was greater for metastatic versus non-metastatic patients (30.1% vs. 23.0%, p < 0.001) (Fig. 3). The median time to an employment decrease was significantly shorter for metastatic versus non-metastatic patients (4.3 vs. 14.7 months (p < 0.001), respectively).
Estimated cumulative proportion of patients experiencing reduction in work schedule. Reduction in work schedule reported among patients with Active Full Time or Active Part Time employment status in the month prior to the index date and ≥ 1 day of enrollment during months 1–3, 4–6, and 7–12. Met metastatic, Non non-metastatic
3.3 Work Loss
The proportion of patients claiming work loss during the first 3 months after index was greater for metastatic versus non-metastatic for STD (47.1% vs. 20.1%, p < 0.001) and LTD (2.3% vs. 0.6%, p < 0.001), but lower for WA (61.9% vs. 74.2%, p < 0.001). A greater proportion of the metastatic cohort claiming STD and LTD was consistent across the remaining time periods (Fig. 4). The proportion of patients claiming LTD increased through the 12-month post-index period, whereas the proportion claiming STD decreased, and the proportion with WA remained constant.
Among patients claiming work loss, the mean number of days lost PPPM during the first 3 months after cancer diagnosis was higher for metastatic versus non-metastatic for WA (5.7 vs. 3.6, p < 0.001), STD (15.4 vs. 11.2, p < 0.001), and LTD (14.3 vs. 13.2, p < 0.05) (Fig. 5). The greater number of days lost among the metastatic cohort versus the non-metastatic cohort was generally consistent across all time periods assessed. The mean number of days lost decreased for WA and STD but remained constant for LTD throughout the 12 months after index.
The mean number of days lost in the 12-month post-diagnosis period aggregating WA, STD, and LTD (Fig. 6) demonstrated increased number of days lost in metastatic versus non-metastatic patients in the 3-, 6-, and 12-month post-diagnosis period. This represents an estimate for time lost on average across patients over a 12-month period due to patient attrition in the first year of the analysis, with smaller numbers of patients contributing to the analysis later in the year. The mean number of days of missed work for metastatic versus non-metastatic patients was 33.39 versus 14.91 (ratio = 2.24), 64.05 versus 27.15 (ratio = 2.36), and 105.93 versus 46.29 (ratio = 2.29) days within 3, 6, and 12 months after cancer diagnosis, respectively.
Figure 7 presents the estimated indirect costs from work loss. Mean indirect costs during the first 12 months after cancer diagnoses, based on the national average wage calculation, were higher for metastatic versus non-metastatic patients by a difference of $10,746 (p < 0.001). The difference ranged from $6,877 to $22,283 based on sensitivity analyses (Fig. 7).
Total mean indirect costs due to WA, STD, and LTD over the first 12 months after cancer diagnoses. LTD long-term disability, Met metastatic, Non non-metastatic, STD short-term disability, WA work absence. p < 0.001 for all metastases vs. non metastases comparisons. Total costs were estimated by summing costs due to WA, STD, and LTD. Low-rate industries wage rate = 68% of the national average rates; high-rate industries wage rate = 162% of the national average rates; full cost to employer was derived based on the national average wage rate with a multiplier of 1.28; full cost to employer for low/high-rate industries are based on low/high-rate industry wage rates with a multiplier of 1.28
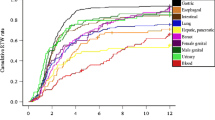
Analyses were repeated for subgroups for patients aged 50 years and older, and for selected types of cancer. The impacts of a metastatic diagnosis on lost days of work were consistent among older patients and patients with specific cancer types (breast, lung, colon, pancreatic, and liver cancers) (Fig. S1 and Fig. S2 in the OSM).
4 Discussion
The results from this study demonstrated that productivity loss and indirect costs are significantly greater among patients diagnosed with cancer at late (i.e., metastatic) versus early (i.e., non-metastatic) stages. The work loss due to work absence, short-term disability, and long-term disability within the first year after cancer diagnosis amounted to 106 workdays for metastatic versus 46 workdays for non-metastatic patients, with a difference of 60 days or nearly a quarter of the total number of working days in 1 year (Fig. 6).
Our results are consistent with prior studies on work loss and indirect cost related to cancer. In particular, the metastatic and non-metastatic estimates of work loss from our study provide an upper and lower range surrounding the results published among all cancer patients. The mean number of days lost PPPM within the first 3 months after cancer diagnosis for metastatic versus non-metastatic was 3.5 versus 2.6 for WA (p < 0.001) and 7.3 versus 2.3 for STD (p < 0.001), respectively, which are similar to the 2.0 WA and 5.0 STD mean days of lost PPPM among all cancer patients reported by Chang et al. [11]. The finding that the proportion of patients claiming work loss was greater for metastatic versus non-metastatic for STD and LTD but slightly lower for WA suggests that patients diagnosed with metastatic cancers require extended period of time off from work due to the severity of the disease and aggressive treatments.
Our finding of late-stage cancer diagnosis resulting in reduced employment status is also supported in the literature. In our study, within the first year after cancer diagnosis, the proportion of metastatic patients with a reduction in work schedule was found to be doubled that of non-metastatic patients, i.e., 22% of metastatic patients versus 11% of non-metastatic patients. This difference was also observed during the full post-index period (30% vs. 23%, p < 0.001), although the substantial variation in duration of follow up between the cohorts complicates the interpretation of this result. In a secondary analysis of clinical trial data, cancer stage (metastatic vs. non-metastatic) was a significant predictor for change in employment status (p < 0.0001) [18]. A survey study of non-metastatic cancer patients reported 24% of patients with some change in employment due to illness, measured more than 6 months from diagnosis in an observational multi-site Symptom Outcomes and Practice Patterns study [10]. This is similar to our finding of 23% of non-metastatic patients with a reduction in work schedule during the full post-index period. While the comparison study was limited in sample size (N = 530), multivariable regression found participants with at least moderate symptom interference were more likely to report “no longer working” [10]. Metastatic cancer patients demonstrate worse symptom severity and physical function than non-metastatic cancer patients, likely driving the higher proportion of these patients with reduction in work schedule observed in our study [19].
This study is subject to limitations common in claims data analysis, including issues such as incomplete recording of clinical data and miscoding. Second, claims data do not contain the pathological findings needed to determine precise cancer stages such as tumor size, so metastasis diagnosis (secondary cancer) was used as a proxy for late (advanced) cancer stage. Additionally, the different lengths of follow-up for metastatic versus non-metastatic cohorts could bias the results. For example, non-metastatic patients had longer follow-up, and thus more opportunity to demonstrate reduction in employment status or incur work loss. To account for these differences, we reported outcomes during the first 3 months and incrementally during the first year after cancer diagnosis. Additionally, the total indirect costs of cancer should consider both employment and work-loss outcomes, but we were not able to integrate the two. Work-loss cohorts for WA, STD, and LTD were selected separately so the results integrating WA, STD, and LTD are an extrapolation from the individual eligibility cohorts. We were also unable to capture other aspects of indirect costs to the employer, including presenteeism of the cancer patients and productivity loss of caregivers, which likely underestimates the total indirect costs of cancer. Finally, this study used data from individuals in the USA with commercial insurance, and the results may not be generalizable to patients outside the USA, or to patients with other insurance or without insurance. The work-loss outcomes—STD and LTD in particular—are only available for employers offering disability insurance programs, and small employers may not have the same benefit rule as large employers if the program is even available. For these reasons, the results of this study cannot be extrapolated to project the productivity loss by cancer stage to the US population.
Our study did not examine indirect cost associated with lost worktime of caregivers, which adds to the total economic impact of lost productivity from cancer. Moore et al. indicate that 25–29% of informal cancer caregivers make extended employment changes [20]. Data from a national survey of caregivers of cancer patients from 2003 to 2006 estimated that the number of months and daily hours spent caregiving were the highest for caregivers of cancer survivors diagnosed with distant disease compared with survivors with regional or localized disease (p < 0.05) [8]. Annual costs to informal caregivers were estimated using the median wage rate in 2006 ($16.28), and amounted to an estimated $36,000 for those caring for lung cancer patients and about $19,000 for those caring for breast cancer patients [8].
More recent data from a cross-sectional survey among 319 lung and breast cancer patients, and their unpaid caregivers, found that a later stage at diagnosis correlated with greater absenteeism [21]. The estimated productivity loss included reduced effectiveness (presenteeism) in addition to time absent from work and was specific to the patient’s reported income, with an estimated annual cost of more than $120,000 for productivity loss [21].
Other approaches to estimate indirect cost include lost productivity due to premature death (mortality cost) estimated via the human capital or willingness-to-pay methods [9]. Using these methods, cancer mortality is associated with $94.4 billion lost in annual earnings [22] and ten more times if including intrinsic value of life lost [23]. In addition to indirect costs, one cannot ignore the medical cost of cancer. The projected total healthcare cost for cancer in 2020 was $157 billion (2010 dollars) based on SEER-Medicare database analyses and cancer prevalence projection [24], which at a patient level is higher among metastatic versus non-metastatic cancer [6].
The value of early cancer detection and screening should adopt a societal perspective by considering the indirect costs in addition to the direct medical costs, because by some estimates, the indirect costs are a majority contributor [9, 25, 26]. Earlier detection can result in more effective and more cost-effective treatment being available to the patient, and reduce the financial impact of cancer, individually and to the broader economy [27].
5 Conclusions
The total economic burden of cancer includes significant indirect costs in addition to direct medical costs. Patients diagnosed with later-stage cancer had significantly greater rates of work reduction, more days absent from work, and higher associated indirect costs for employers. Earlier cancer detection may reduce the disruptive effects of cancer on the life and work of patients and attenuate the economic burden to employers and society by initiating treatment before cancer progresses to late stage.
References
Centers for Disease Control and Prevention. An update on cancer deaths in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control; 2021.
Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364: l408.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Howlander N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019.
Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2019) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, released February 2021.
McGarvey N, Gitlin M, Qi J, Chung KC. Increasing healthcare costs by stage and over time among patients diagnosed with cancer: 2006–2020. AMCP NEXUS; October 18–21, 2021.
Kakushadze Z, Raghubanshi R, Yu W. Estimating cost savings from early cancer diagnosis. Data. 2017;2:30. https://doi.org/10.3390/data2030030.
Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–73. https://doi.org/10.1002/cncr.24588.
Yabroff KR, Lund J, Kepka D, Mariotto A. Economic burden of cancer in the United States: estimates, projections, and future research. Cancer Epidemiol Biomark Prev. 2011;20:2006–14. https://doi.org/10.1158/1055-9965.EPI-11-0650.
Tevaarwerk AJ, Lee JW, Sesto ME, et al. Employment outcomes among survivors of common cancers: the symptom outcomes and practice patterns (SOAPP) study. J Cancer Surviv. 2013;7:191–202. https://doi.org/10.1007/s11764-012-0258-2.
Chang S, Long SR, Kutikova L, et al. Estimating the cost of cancer: results on the basis of claims data analyses for cancer patients diagnosed with seven types of cancer during 1999 to 2000. J Clin Oncol. 2004;22:3524–30. https://doi.org/10.1200/JCO.2004.10.170.
Kamal KM, Covvey JR, Dashputre A, et al. A systematic review of the effect of cancer treatment on work productivity of patients and caregivers. J Manag Care Spec Pharm. 2017;23:136–62. https://doi.org/10.18553/jmcp.2017.23.2.136.
Adamson, D.; Chang, S.; Hansen, L. Health Research Data for the Real World: The MarketScan Databases; Thomson Medstat: New York, NY, USA, 2006, http://patientprivacyrights.org/wp-content/uploads/2011/06/Thomson-Medstat-white-paper.pdf. Accessed 10 Aug 2022.
Hightower S. The basics of short-term disability insurance. https://www.insure.com/disability-insurance/short-term-disability.html. Accessed 16 Nov 2021.
Bureau of the Census. Current population survey, 2020 annual social and economic (ASEC) supplement conducted by the Bureau of the Census for the Bureau of Labor Statistics. Washington, DC: Bureau of the Census; 2020.
Bureau of Labor Statistics. https://www.bls.gov/news.release/empsst.t19.htm. Accessed 15 Dec 2021.
Nicholson S, Pauly MV, Polsky D, Baase CM, Billotti GM. How to present the business case for healthcare quality to employers. Appl Health Econ Health Policy. 2005;4:209–18. https://doi.org/10.2165/00148365-200504040-00003.
Tevaarwerk A, Lee J, Sesto MC, et al. Predictors of employment (empl) outcomes in outpatients (pts) with common solid tumors: a secondary analysis from E2Z02 (ECOG’s SOAPP study). J Clin Oncol. 2010;28:9118. https://doi.org/10.1200/jco.2010.28.15_suppl.9118.
Siddiqi A, Given CW, Given B, Sikorskii A. Quality of life among patients with primary, metastatic and recurrent cancer. Eur J Cancer Care. 2009;18:84–96.
de Moor JS, Dowling EC, Ekwueme DU, et al. Employment implications of informal cancer caregiving. J Cancer Surviv. 2017;11:48–57. https://doi.org/10.1007/s11764-016-0560-5.
May SG, Chiu K, MacEwan JP, et al. The impact of a cancer diagnosis on worker productivity: Results from a survey of cancer patients and caregivers. J Clin Oncol. 2020;38:144. https://doi.org/10.1200/JCO.2020.38.29_suppl.144.
Islami F, Miller KD, Siegel RL, et al. National and state estimates of lost earnings from cancer deaths in the United States. JAMA Oncol. 2019;5:e191460. https://doi.org/10.1001/jamaoncol.2019.1460.
Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst. 2008;100:1755–62. https://doi.org/10.1093/jnci/djn383.
Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomark Prev. 2020;29:1304–12. https://doi.org/10.1158/1055-9965.EPI-19-1534.
Yabroff KR, Mariotto AM, Tangka F, et al. Annual report to the nation on the status of cancer, part II: patient economic burden associated with cancer care. JNCI. 2021. https://doi.org/10.1093/jnci/djab192.
Huang SY, Chen HM, Liao KH, Ko BS, Hsiao FY. Economic burden of cancers in Taiwan: a direct and indirect cost estimate for 2007–2017. J Natl Cancer Inst. 2020;10(10): e036341. https://doi.org/10.1136/bmjopen-2019-036341.
Tafazzoli A, et al. Cost-effectiveness of multi-cancer early detection (MCED) test in subgroups with smoking history or obesity. JNCCN. 2022. https://doi.org/10.6004/jnccn.2021.7244.
Acknowledgements
Medical writing services were provided by Meghan Moynihan, an employee of IBM Watson Health, who developed the first draft of the manuscript. Programming services were provided by Laurie Costa, an employee of IBM Watson Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Grail LLC, a subsidiary of Illumina, Inc.
Conflicts of interest
Ze Cong and Karen Chung are employed by GRAIL, LLC, a subsidiary of Illumina, Inc., Menlo Park, CA, USA* *GRAIL, LLC is currently held separate from Illumina Inc. under the terms of the Interim Measures Order of the European Commission dated 29 October 2021, and hold stock in Illumina, Inc. Oth Tran, James Nelson, and Monica Silver were or are employed by IBM Watson Health, which received funding from Grail LLC, a subsidiary of Illumina, Inc., to conduct this study.
Availability of data and material
The data that support the findings of this study are available from IBM Watson Health. Restrictions apply to the availability of these data, which were used under license for this study.
Authors’ contributions
All authors contributed to the formulation and design of the study, preparation of the manuscript, and approved the final manuscript. OT, JN, and MS conducted the analyses.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication (from patients/participants)
Not applicable.
Code availability
The codes generated during the current study are available from the corresponding author on reasonable request.
Additional information
GRAIL, LLC is currently held separate from Illumina Inc. under the terms of the Interim Measures Order of the European Commission dated 29 October 2021.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cong, Z., Tran, O., Nelson, J. et al. Productivity Loss and Indirect Costs for Patients Newly Diagnosed with Early- versus Late-Stage Cancer in the USA: A Large-Scale Observational Research Study. Appl Health Econ Health Policy 20, 845–856 (2022). https://doi.org/10.1007/s40258-022-00753-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-022-00753-w