Abstract
Background
CT-P13 subcutaneous (SC)—the first and only SC version of infliximab—is approved by the European Medicines Agency for the treatment of rheumatoid arthritis (RA). This new mode of infliximab administration will allow patients to self-inject at home, significantly reducing the number of outpatient visits and costs of intravenous (IV) administration. This paper describes the economic impact of introducing CT-P13 SC to the market from the UK societal perspective.
Objective
The budget impact analysis was conducted to assess the financial impact of the adoption of CT-P13 SC over a 5-year period.
Methods
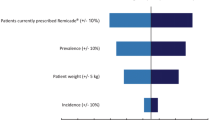
A prevalence-based budget impact model was developed incorporating epidemiological data, administration cost data, and market share data. The analysis compared a “world with” CT-P13 SC scenario to a “world without” CT-P13 SC. A sensitivity analysis included dose escalation up to 4.1 mg/kg to reflect the real-world care delivery setting.
Results
Compared to the “world without” scenario, the introduction of CT-P13 SC resulted in cost savings of ₤69.3 million in the UK over a 5-year period. In the scenario analysis, the saving increased to ₤173.5 million over 5 years.
Conclusion
Use of CT-P13 SC may lead to substantial cost savings for the UK society.






Similar content being viewed by others
References
The Burden of Rheumatoid Arthritis across Europe: a Socioeconomic Survey (BRASS): British Society of Rheumatology.
A D-G. Rheumatoid arthritis. 2019. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Rheumatoid-Arthritis. Cited 24 August 2020.
(NRAS) NRAS. What is RA? https://www.nras.org.uk/what-is-ra-article#:~:text=About%201%25%20of%20the%20population,a%20bit%20older%20for%20men. Cited 27 July 2020.
Institute for Health Metrics and Evaluation. GBD results tool. Global burden of disease data resources. 2017. http://ghdx.healthdata.org/gbd-results-tool.
Freeman J. RA facts: what are the latest statistics on rheumatoid arthritis? 2018. https://www.rheumatoidarthritis.org/ra/facts-and-statistics/. Cited 25 Aug 2020.
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Prim. 2018;4(1):18001.
Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology. 2000;39(2):122–32.
Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum. 2003;48(1):59–63.
Matcham F, Scott IC, Rayner L, Hotopf M, Kingsley GH, Norton S, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–30.
Tuominen R, Tuominen S, Suominen C, Möttönen T, Azbel M, Hemmilä J. Perceived functional disabilities among rheumatoid arthritis patients. Rheumatol Int. 2010;30(5):643–9.
Leino M, Tuominen S, Pirilä L, Tuominen R. Effects of rheumatoid arthritis on household chores and leisure-time activities. Rheumatol Int. 2015;35(11):1881–8.
Wikström I, Book C, Jacobsson LT. Difficulties in performing leisure activities among persons with newly diagnosed rheumatoid arthritis: a prospective, controlled study. Rheumatology (Oxford). 2006;45(9):1162–6.
GBD Results Tool. Institute for Health Metrics and Evaluation.
Chronic rheumatic conditions. https://www.who.int/chp/topics/rheumatic/en/. Cited 25 Aug 2020.
Living longer: how our population is changing and why it matters. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/articles/livinglongerhowourpopulationischangingandwhyitmatters/2018-08-13. Cited 25 Aug 2020.
Morgan E. National life tables, UK: 2016 to 2018. 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2016to2018. Cited 25 Aug 2020.
Belloni BFA. Ageing and health expenditure. 2019. https://publichealthmatters.blog.gov.uk/2019/01/29/ageing-and-health-expenditure/. Cited 25 Aug 2020.
Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–77.
Papadopoulos CG, Gartzonikas IK, Pappa TK, Markatseli TE, Migkos MP, Voulgari PV, et al. Eight-year survival study of first-line tumour necrosis factor α inhibitors in rheumatoid arthritis: real-world data from a university centre registry. Rheumatol Adv Pract. 2019;3(1):rkz007.
Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54(4):1075–86.
Perdriger A. Infliximab in the treatment of rheumatoid arthritis. Biologics. 2009;3:183–91.
Agency EM. Remicade. Infliximab in the treatment of rheumatoid arthritis. 2019. Cited 25 Aug 2020.
Holroyd CR, Parker L, Bennett S, Zarroug J, Underhill C, Davidson B, et al. Switching to biosimilar infliximab: real world data in patients with severe inflammatory arthritis. Clin Exp Rheumatol. 2018;36(1):171–2.
Moots RJ, Curiale C, Petersel D, Rolland C, Jones H, Mysler E. Efficacy and safety outcomes for originator TNF inhibitors and biosimilars in rheumatoid arthritis and psoriasis trials: a systematic literature review. BioDrugs. 2018;32(3):193–9.
Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology. 2012;51(suppl_6):vi28–36.
Schwartzman S, Morgan GJ, Jr. Does route of administration affect the outcome of TNF antagonist therapy? Arthritis Res Ther. 2004;6(Suppl 2):S19–23.
Janssen Biologics B.V. Remicade 100 mg powder for concentrate for solution for infusion. [Summary of Product Characteristics]. European medicines agency website. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/remicade. Accessed 25 Aug 2020.
Celltrion Healthcare Hungary Kft. Remsima 100 mg powder for concentrate for solution for infusion [Summary of Product Characteristics]. European medicines agency website. 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/remsima#product-information-section. Accessed 24 Aug 2020.
Schmier J, Ogden K, Nickman N, Halpern MT, Cifaldi M, Ganguli A, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–17.
Tetteh EK, Morris S. Evaluating the administration costs of biologic drugs: development of a cost algorithm. Health Econ Rev. 2014;4(1):26.
Soini EJ, Leussu M, Hallinen T. Administration costs of intravenous biologic drugs for rheumatoid arthritis. Springerplus. 2013;2:531.
Sørensen J, Linde L, Hetland ML. Contact frequency, travel time, and travel costs for patients with rheumatoid arthritis. Int J Rheumatol. 2014;2014:285951.
Fautrel B, Woronoff-Lemsi MC, Ethgen M, Fein E, Monnet P, Sibilia J, et al. Impact of medical practices on the costs of management of rheumatoid arthritis by anti-TNFalpha biological therapy in France. Jt Bone Spine. 2005;72(6):550–6.
Walsh CA, Minnock P, Slattery C, Kennedy N, Pang F, Veale DJ, et al. Quality of life and economic impact of switching from established infliximab therapy to adalimumab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46(7):1148–52.
Stoner KL, Harder H, Fallowfield LJ, Jenkins VA. Intravenous versus subcutaneous drug administration which do patients prefer? A systematic review. Patient Patient Cent Outcomes Res. 2015;8(2):145–53.
Navarro-Millán I, Herrinton LJ, Chen L, Harrold L, Liu L, Curtis JR. Comparative effectiveness of etanercept and adalimumab in patient reported outcomes and injection-related tolerability. PLoS ONE. 2016;11(3):e0149781.
Huynh TK, Ostergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adher. 2014;8:93–9.
Ariza-Ariza R, Navarro-Sarabia F, Hernández-Cruz B, Rodríguez-Arboleya L, Navarro-Compán V, Toyos J. Dose escalation of the anti-TNF-α agents in patients with rheumatoid arthritis. A systematic review. Rheumatology. 2006;46(3):529–32.
Rahman MU, Strusberg I, Geusens P, Berman A, Yocum D, Baker D, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66(9):1233–8.
Westhovens R, Wiland P, Zawadzki M, Ivanova D, Kasay AB, El-Khouri EC, et al. Efficacy, pharmacokinetics and safety of subcutaneous versus intravenous CT-P13 in rheumatoid arthritis: a randomized phase I/III trial. Rheumatology (Oxford). 2020;60(5):2277–2287
Khan S, Rupniewska E, Neighbors M, Singer D, Chiarappa J, Obando C. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J Clin Pharm Ther. 2019;44(4):495–507.
Salaffi F, Di Carlo M, Farah S, Carotti M. Adherence to subcutaneous anti-TNFα agents in patients with rheumatoid arthritis is largely influenced by pain and skin sensations at the injection site. Int J Rheum Dis. 2020;23(4):480–7.
Bhoi P, Bessette L, Bell MJ, Tkaczyk C, Nantel F, Maslova K. Adherence and dosing interval of subcutaneous antitumour necrosis factor biologics among patients with inflammatory arthritis: analysis from a Canadian administrative database. BMJ Open. 2017;7(9):e015872.
Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
Clare DF, Alexander FC, Mike S, Dan G, Allan F, Lisa W, et al. Accelerated infliximab infusions are safe and well tolerated in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21(1):71–5.
McConnell J, Parvulescu-Codrea S, Behm B, Hill B, Dunkle E, Finke K, et al. Accelerated infliximab infusions for inflammatory bowel disease improve effectiveness. World J Gastrointest Pharmacol Ther. 2012;3(5):74–82.
(ONS) OfNS. United Kingdom population mid-year estimate. 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Accessed 10 Oct 2020.
Heald A, Bramham-Jones S, Davies M. Comparing cost of intravenous infusion and subcutaneous biologics in COVID-19 pandemic care pathways for rheumatoid arthritis and inflammatory bowel diseases—a brief UK stakeholder survey. Int J Clin Pract. 2021;e14341.
Consumer price inflation tables. In: Statistics OfN, editor. Office for National Statistics; 2020.
(ONS) OfNS. Average actual weekly hours of work for full-time workers (seasonally adjusted). 2020. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/timeseries/ybuy/lms. Cited 4 Nov 2020.
(ONS) OfNS. AWE: whole economy level (£): seasonally adjusted total pay excluding arrears. 2020. https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/timeseries/kab9/emp. Cited 4 Nov 2020.
N NRAS. ‘I Want to Work’ Survey. 2014.
Takeuchi T, Miyasaka N, Tatsuki Y, Yano T, Yoshinari T, Abe T, et al. Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(7):1208–15.
Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(5):704–7.
Caporalia R, Allanore Y, Altenc R, Combed B, Dureze P, Iannonef F, Nurmohamedg MT, Leei SJ, Kwonj TS, Choij JS, Parki G, Yook DH. Efficacy and safety of infliximab subcutaneous versus adalimumab, etanercept and infliximab intravenous in patients with rheumatoid arthritis: a systematic literature review and treatment comparison. 2020;17(1):85–99.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by Celltrion Healthcare.
Conflict of interest
HGB, MYJ, HKY, JP, and TSK report that they have a financial interest in Celltrion Healthcare, a company that may be affected by the research reported in the enclosed paper. All authors are employed by Celltrion Healthcare.
Availability of data and material
All data analysed during this study are included in this published article and its Supplementary Table file.
Code availability
The model is available on request.
Authors’ contributions
Conceptualisation: TSK, MYJ; original draft: HGB, MYJ; editing: HGB, MYJ; data analysis: MYJ, HKY; review: TSK, HKY, JP; supervision: TSK
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Byun, H.G., Jang, M., Yoo, H.K. et al. Budget Impact Analysis of the Introduction of Subcutaneous Infliximab (CT-P13 SC) for the Treatment of Rheumatoid Arthritis in the United Kingdom. Appl Health Econ Health Policy 19, 735–745 (2021). https://doi.org/10.1007/s40258-021-00673-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-021-00673-1