Abstract
Objectives
This study aimed to provide an exhaustive description of criteria and methodological recommendations for evaluating them in health technology assessment (HTA) in Western and Asian countries.
Methods
We conducted a system literature review of HTA-related guidelines by searching the websites of HTA agencies and related data sources. The guidelines, reports, or recommendations introducing the HTA evaluation methods, processes, decision-making frameworks, and criteria for priority setting were eligible to be included. The review was limited to guidelines from countries belonging to the European Network for Health Technology Assessment (EUnetHTA) and HTAsiaLink organisations and other countries with well-established available guidelines.
Results
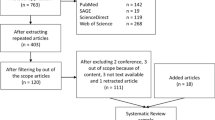
A total of 52 guidelines from 24 countries were identified, including 13 countries from the EUnetHTA organisation, 9 countries from the HTAsiaLink organisation and 2 other countries (Canada and the USA). A strong consensus was observed among the HTA agencies on the core set of criteria including efficacy or effectiveness, cost-effectiveness, safety, and budget impact. More similarities were observed than differences in methodological recommendations for clinical and economic evaluations among the agencies.
Conclusions
Substantial convergence is seen in the criteria included in the HTA process, as well as the methods to evaluate and quantify them. Further efforts are needed to verify whether the criteria identified from the guidelines are incorporated in real HTA decisions, and how they are assessed and weighted in practice.
Similar content being viewed by others
References
Eunethta. EUnetHTA comments on the discussion document: “Health in Europe: A Strategic Approach”. 2007. https://www.eunethta.eu/wp-content/uploads/2018/01/EUnetHTAs-comments-on-the-Health-Strategy.pdf. Accessed Jan 2020.
Frazão TDC, Camilo DGG, Cabral ELS, Souza RP. Multicriteria decision analysis (MCDA) in health care: a systematic review of the main characteristics and methodological steps. BMC Med Inform Decis Mak. 2018;18(1):90. https://doi.org/10.1186/s12911-018-0663-1.
EUnetHTA JA2 WP8 DELIVERABLE HTA Core Model Version 3.0, 25 Jan 2016. https://eunethta.eu/wp-content/uploads/2018/03/HTACoreModel3.0-1.pdf. Accessed Jan 2020.
Evidence and value impact on decision making (EVIDEM).10th Edition concept & definitions. 2017. https://www.evidem.org/wp/wp-content/uploads/2017/09/EVIDEM-10th-Edition-Concept-and-definitions.pdf. Accessed Jan 2020.
Rehfuess EA, Stratil JM, Scheel IB, Portela A, Norris SL, Baltussen R. The WHO-INTEGRATE evidence to decision framework version 1 0: integrating WHO norms and values and a complexity perspective. BMJ Glob Health. 2019;4(Suppl 1):e000844. https://doi.org/10.1136/bmjgh-2018-000844.
Epstein RS, Sidorov J, Lehner JP, Salimi T. Integrating scientific and real-world evidence within and beyond the drug development process. J Comp Eff Res. 2012;1(1 Suppl):9–13. https://doi.org/10.2217/cer.11.3.
Hao Y, Thomas A. Health technology assessment and comparative effectiveness research: a pharmaceutical industry perspective. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):447–54. https://doi.org/10.1586/14737167.2013.815401.
Friedmann C, Levy P, Hensel P, Hiligsmann M. Using multi-criteria decision analysis to appraise orphan drugs: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2018;18(2):135–46. https://doi.org/10.1080/14737167.2018.1414603.
Guindo LA, Wagner M, Baltussen R, Rindress D, van Til J, Kind P, et al. From efficacy to equity: Literature review of decision criteria for resource allocation and healthcare decisionmaking. Cost Eff Resour Alloc. 2012;10(1):9. https://doi.org/10.1186/1478-7547-10-9.
Mobinizadeh M, Raeissi P, Nasiripour AA, Olyaeemanesh A, Tabibi SJ. The health systems’ priority setting criteria for selecting health technologies: a systematic review of the current evidence. Med J Islam Repub Iran. 2016;30:329.
Morgan RL, Kelley L, Guyatt GH, Johnson A, Lavis JN. Decision-making frameworks and considerations for informing coverage decisions for healthcare interventions: a critical interpretive synthesis. J Clin Epidemiol. 2018;94:143–50. https://doi.org/10.1016/j.jclinepi.2017.09.023.
Stratil JM, Baltussen R, Scheel I, Nacken A, Rehfuess EA. Development of the WHO-INTEGRATE evidence-to-decision framework: an overview of systematic reviews of decision criteria for health decision-making. Cost Eff Resour Alloc. 2020;18:8. https://doi.org/10.1186/s12962-020-0203-6.
Specchia ML, Favale M, Di Nardo F, Rotundo G, Favaretti C, Ricciardi W, et al. How to choose health technologies to be assessed by HTA? A review of criteria for priority setting. Epidemiol Prev. 2015;39(4 Suppl 1):39–44.
Zelei T, Molnár MJ, Szegedi M, Kaló Z. Systematic review on the evaluation criteria of orphan medicines in Central and Eastern European countries. Orphanet J Rare Dis. 2016;11(1):72. https://doi.org/10.1186/s13023-016-0455-6.
Xcenda: integration of patient-reported outcomes (PROs) and the patient voice: a review of 6 health technology assessment (HTA) agencies. https://www.xcenda.com/insights/htaq-spring-2020-pro-patient-voice-hta. Accessed Mar 2020.
Liu G, Wu EQ, Ahn J, Kamae I, Xie J, Yang H. The development of health technology assessment in Asia: current status and future trends. Value Health Reg Issues. 2020;21:39–44. https://doi.org/10.1016/j.vhri.2019.08.472.
Sivalal S. Health technology assessment in the Asia Pacific region. Int J Technol Assess Health Care. 2009;25(Suppl 1):196–201. https://doi.org/10.1017/s0266462309090631.
HTAsiaLink. Overview. https://www.htasialink.org/about/overview.html. Accessed Jan 2020.
Indonesian Health Technology Assessment Committee. Health Technology Assessment (HTA) Guideline. 2017. http://www.gear4health.com/uploads/files/file-15-5a441f38c8992.pdf. Accessed Jan 2020.
Department of Health-Philippines. Philippine Methods Guide for Health Technology Assessment First Edition. 2020. https://www.doh.gov.ph/sites/default/files/health_advisory/HTA%20Methods%20Guide_Public%20Consultation%2003.2020_1.pdf. Accessed Apr 2020.
Agency for care effectiveness. Drug evaluation methods and process guide Version 2.0. 2019. http://www.ace-hta.gov.sg/public-data/our-process-and-methods/ACE%20methods%20and%20process%20guide%20for%20medical%20technologies%20evaluation%20(1%20Oct%202018).pdf. Accessed Jan 2020.
Medical association of Thailand. Guidelines for Health Technology Assessment in Thailand (Second Edition). 2014. http://www.hitap.net/wp-content/uploads/2017/06/Thai-HTA-guideline-UPDATES-Jmed-with-Cover.pdf. Accessed Jan 2020.
EUnetHTA. The EUnetHTA network. https://www.eunethta.eu/the-eunethta-network/. Accessed Jan 2020.
HTAsiaLink. HTAsiaLink members. https://www.htasialink.org/member/HTAsiaLinkMembers.html. Accessed Jan 2020.
HTAsiaLink. https://htasialink2020.com. Accessed Aug 2020.
ISPOR. The ISPOR Global Health Care Systems road map. https://tools.ispor.org/htaroadmaps/. Accessed Jan 2020.
EUnetHTA. Relative effectiveness assessment (REA) of pharmaceuticals. 2011. https://www.eunethta.eu/wp-content/uploads/2018/01/Final-version-of-Background-Review-on-Relative-Effectiveness-Assessmentappendix.pdf. Accessed Jan 2020.
EUnetHTA. Methods for health economic evaluations—a guideline based on current practices in Europe. 2015. https://www.eunethta.eu/wpcontent/uploads/2018/03/Methods_for_health_economic_evaluations.pdf. Accessed Jan 2020.
ISPOR. Pharmacoeconomic guidelines around the world. https://tools.ispor.org/peguidelines/#:~:text=Pharmacoeconomic%20(PE)%20guidelines%20can%20be,evaluating%20the%20economic%20study%20reports. Accessed Jan 2020.
Dunlop WC, Mullins CD, Pirk O, Goeree R, Postma MJ, Enstone A, et al. BEACON: a summary framework to overcome potential reimbursement hurdles. PharmacoEconomics. 2016;34(10):1051–65. https://doi.org/10.1007/s40273-016-0427-7.
Heintz E, Gerber-Grote A, Ghabri S, Hamers FF, Rupel VP, Slabe-Erker R, et al. Is there a European view on health economic evaluations? Results from a synopsis of methodological guidelines used in the EUnetHTA partner countries. PharmacoEconomics. 2016;34(1):59–76. https://doi.org/10.1007/s40273-015-0328-1.
Kleijnen S, George E, Goulden S, d’Andon A, Vitré P, Osińska B, et al. Relative effectiveness assessment of pharmaceuticals: similarities and differences in 29 jurisdictions. Value Health. 2012;15(6):954–60. https://doi.org/10.1016/j.jval.2012.04.010.
Belgian Health Care Knowledge Centre. Incorporating societal preferences in reimbursement decisions. 2014. https://kce.fgov.be/sites/default/files/atoms/files/KCE_234_reimbursement_decisions_Report_0.pdf. Accessed Jan 2020.
Belgian Health Care Knowledge Centre. Drug reimbursement systems: international comparison and policy recommendations. 2010. https://kce.fgov.be/sites/default/files/atoms/files/KCE_147C_Drug_reimbursement_systems_4.pdf. Accessed Jan 2020.
Belgian Health Care Knowledge Centre. Belgian guidelines for economic evaluations and budget impact analyses: second edition. 2015. https://kce.fgov.be/en/belgian-guidelines-for-economic-evaluations-and-budget-impact-analyses-second-edition. Accessed Jan 2020.
Agency for Quality and Accreditation in Health Care. The Croatian guideline for health technology assessment process and reporting. 2011. http://www.aaz.hr/sites/default/files/hrvatske_smjernice_za_procjenu_zdravstvenih_tehnologija.pdf. Accessed Jan 2020.
Finnish Medicines Agency. Rapid assessment of new hospital-only medicinal products. 2018. https://www.fimea.fi/documents/542809/2233402/170821_Sairaalal%C3%A4%C3%A4kkeiden_arviointi_prosessi_EN.pdf/63bc259d-b170-2cf2-e884-bb813ba8e4a6. Accessed Jan 2020.
Health Insurance Review and Assessment Service. Preparing a health economic evaluation to be attached to the application for reimbursement status and wholesale price for a medicinal product. 2019. https://www.hila.fi/content/uploads/2020/01/Instructions_TTS_2019.pdf. Accessed Jan 2020.
French National Authority for Health. Methods and criteria for assessing medicinal products. 2015. https://www.has-sante.fr/jcms/c_2035651/en/methods-and-criteria-for-assessing-medicinal-products. Accessed Jan 2020.
French National Authority for Health. Principles of medicinal products assessment and appraisal for reimbursement purposes. 2019. https://www.has-sante.fr/upload/docs/application/pdf/2019-07/doctrine_de_la_commission_de_la_transparence_-_version_anglaise.pdf. Accessed Jan 2020.
Commission for Economic Evaluation and Public Health. Choices in methods for economic evaluation. 2012. https://www.has-sante.fr/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf. Accessed Jan 2020.
Ghabri S, Autin E, Poullié AI, Josselin JM. The French National Authority for Health (HAS) guidelines for conducting budget impact analyses (BIA). PharmacoEconomics. 2018;36(4):407–17. https://doi.org/10.1007/s40273-017-0602-5.
Institute for Quality and Efficiency in Health Care. General methods version 5.0 of 10 Jul 2017. https://www.iqwig.de/en/methods/methods-paper.3020.html. Accessed Jan 2020.
National Institute of Pharmacy and Nutrition. Professional healthcare guideline on the methodology of health technology assessment. 2017. https://tools.ispor.org/PEguidelines/source/HTA_Guideline_HUN_eng.pdf. Accessed Jan 2020.
Health Information and Quality Authority. A guide to health technology assessment at HIQA. 2016. https://www.hiqa.ie/sites/default/files/2017-01/A-Guide-to-Health-Technology-Assessment.pdf. Accessed Jan 2020.
Health Information and Quality Authority. Guidelines for evaluating the clinical effectiveness of health technologies in Ireland. 2018. https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-evaluating-clinical-effectiveness. Accessed Jan 2020.
Health Information and Quality Authority. Guidelines for the economic evaluation of health technologies in Ireland. 2019. https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-economic-evaluation-health. Accessed Jan 2020.
Health Information and Quality Authority. Guidelines for the budget impact analysis of health technologies in Ireland. 2019. https://www.hiqa.ie/reports-and-publications/health-technology-assessment/guidelines-budget-impact-analysis-health. Accessed Jan 2020.
Norwegian Ministry of Health and Care Services. Principles for priority setting in health care. 2017. https://www.regjeringen.no/contentassets/439a420e01914a18b21f351143ccc6af/en-gb/pdfs/stm201520160034000engpdfs.pdf. Accessed Jan 2020.
Norwegian Medicines Agency. Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals. 2018. https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%20151018.pdf. Accessed Jan 2020.
The Agency for Health Technology Assessment and Tariff System. Health technology assessment guidelines version 3.0. 2016. https://www.aotm.gov.pl/www/wp-content/uploads/wytyczne_hta/2016/20161104_HTA_Guidelines_AOTMiT.pdf. Accessed Jan 2020.
Dental and Pharmaceutical Benefits Agency. Guide for companies when applying for subsidies and pricing for pharmaceutical products version 2.0. 2012. https://heatinformatics.com/sites/default/files/images-videosFileContent/Sweden%20TLV%20Reimbursement%202013.pdf. Accessed Jan 2020.
Swedish Council on Health Technology Assessment. Assessment of methods in health care and social services: a handbook. 2018. https://www.sbu.se/contentassets/76adf07e270c48efaf67e3b560b7c59c/eng_metodboken.pdf. Accessed Jan 2020.
Pharmaceutical Benefits Board. General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2). 2003. https://www.tlv.se/download/18.2e53241415e842ce95514e9/1510316396792/Guidelines-for-economic-evaluations-LFNAR-2003-2.pdf. Accessed Jan 2020.
Institute for innovation & valuation in health care. SWISS HTA CONSENSUS GUIDING PRINCIPLES. 2012. http://www.swisshta.org/index.php/Consensus.html. Accessed Jan 2020.
The Health Care Insurance Board. Assessment of the reimbursement criterion ‘‘current medical science and practice. 2007. https://english.zorginstituutnederland.nl/publications/reports/2007/11/05/assessment-of-the-reimbursement-criterion-%E2%80%98current-medical-science-and-practice%E2%80%99. Accessed Jan 2020.
The Health Care Insurance Board. Dutch Assessment procedures for the reimbursement of outpatient medicines pharmaceutical managementagency. 2010. https://english.zorginstituutnederland.nl/publications/reports/2010/03/01/dutch-assessment-procedures-for-the-reimbursement-of-outpatient-medicines. Accessed Jan 2020.
National Health Care Institute. Guideline for economic evaluations in healthcare. 2016. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare. Accessed Jan 2020.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed Jan 2020.
Scottish Medicines Consortium. A guide to the Scottish Medicines Consortium. https://www.scottishmedicines.org.uk/media/3574/20180712-a-guide-to-the-scottish-medicines-consortium.pdf. Accessed Jan 2020.
All Wales Medicines Strategy Group. Guidance on appraisal structure and evidence considered. 2019. http://www.awmsg.org/docs/awmsg/appraisaldocs/inforandforms/AWMSG%20summary%20guidelines%20for%20appraising%20medicines.pdf. Accessed Jan 2020.
Scottish Medicines Consortium. Working with SMC—a guide for manufacturers. 2017. https://www.scottishmedicines.org.uk/media/3020/working-with-smc.pdf. Accessed Jan 2020.
Scottish Medicines Consortium. Guidance to submitting companies for completion of New Product Assessment Form. 2020. https://www.scottishmedicines.org.uk/media/4990/guidance-supplement-ultra-orphan-updated-011119.pdf. Accessed Jan 2020.
Pharmaceutical Benefits Advisory Committee. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee Version 5.0. 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf. Accessed Jan 2020.
Center for Drug Evaluation. The statement for the process of health technology assessment. Published on Jul 2018. https://www3.cde.org.tw/Content/Files/HTA/1/1_%E5%81%A5%E4%BF%9D%E7%B5%A6%E4%BB%98%E5%BB%BA%E8%AD%B0%E6%9B%B8%E4%B9%8B%EF%A7%81%E6%95%88%E8%A9%95%E4%BC%B0%E7%B0%A1%E8%A6%81%EF%A5%AF%E6%98%8E_V1.pdf. Accessed Jan 2020.
Center for Drug Evaluation. Guidelines of methodological standards for pharmacoeconomic evaluations version 1.1. 2016. https://www3.cde.org.tw/Content/Files/HTA/3/7_PE%E8%A9%95%E4%BC%B0%E6%96%B9%E6%B3%95%E5%AD%B8%E6%8C%87%E5%8D%97.pdf. Accessed Jan 2020.
Center for Drug Evaluation. HTA guidelines of methodological standards for system literature review. 2016. https://www3.cde.org.tw/Content/Files/HTA/3/4_%E9%86%AB%E7%99%82%E7%A7%91%E6%8A%80%E8%A9%95%E4%BC%B0%E6%96%B9%E6%B3%95%E5%AD%B8%E6%8C%87%E5%BC%95%E7%B3%BB%E7%B5%B1%E6%80%A7%E5%9B%9E%E9%A1%A7.pdf. Accessed Jan 2020.
Center for Drug Evaluation.HTA guidelines of methodological standards for budget impact analysis. 2016. https://www3.cde.org.tw/Content/Files/HTA/3/2_%E9%86%AB%E7%99%82%E7%A7%91%E6%8A%80%E8%A9%95%E4%BC%B0%E6%96%B9%E6%B3%95%E5%AD%B8%E6%8C%87%E5%BC%95BIA.pdf. Accessed Jan 2020.
Center for Drug Evaluation.HTA guidelines of methodological standards for cost effectiveness analysis. 2016. https://www3.cde.org.tw/Content/Files/HTA/3/3_%E9%86%AB%E7%99%82%E7%A7%91%E6%8A%80%E8%A9%95%E4%BC%B0%E6%96%B9%E6%B3%95%E5%AD%B8%E6%8C%87%E5%BC%95CEA.pdf. Accessed Jan 2020.
National Evidence-based Healthcare Collaboration Agency. Priority setting for health technology assessment (HTA) research in Korea. 2015. https://www.neca.re.kr/eng/lay1/program/S120T140C141/report/view.do?seq=106. Accessed Jan 2020.
Bae S, Lee S, Bae EY, Jang S. Korean guidelines for pharmacoeconomic evaluation (second and updated version): consensus and compromise. PharmacoEconomics. 2013;31(4):257–67. https://doi.org/10.1007/s40273-012-0021-6.
Malaysian Health Technology Assessment Section. Health technology assessment manual. http://www.moh.gov.my/moh/resources/HTA_MANUAL_MAHTAS.pdf?mid=636. Accessed Jan 2020.
Ministry of Health. Pharmacoeconomic guidelines for Malaysia second edition. 2019. https://www.pharmacy.gov.my/v2/en/documents/pharmacoeconomic-guideline-malaysia-2nd-edition.html. Accessed Jan 2020.
Pharmaceutical Management Agency. Guidelines for funding applications to PHARMAC. 2017. https://www.pharmac.govt.nz/assets/guidelines-for-funding-applications-2017-09.pdf. Accessed Jan 2020.
Pharmaceutical Management Agency. Prescription for pharmacoeconomic analysis—methods for cost-utility analysis. 2015. https://www.pharmac.govt.nz/medicines/how-medicines-are-funded/economic-analysis/pfpa/. Accessed Jan 2020.
Institute for Clinical and Economic Review. A guide to ICER’s methods for health technology assessment. 2018. https://icer-review.org/methodology/icers-methods/icer-hta-guide_082018/. Accessed Jan 2020.
Institute for Clinical and Economic Review. ICER’s reference case for economic evaluations: principles and rationale. 2018. http://icer-review.org/wp-content/uploads/2018/07/ICER_Reference_Case_July-2018.pdf. Accessed Jan 2020.
The Canadian Agency for Drugs and Technologies in Health. pCODR Expert Review Committee Deliberative Framework. 2016. https://www.cadth.ca/sites/default/files/pcodr/The%20pCODR%20Expert%20Review%20Committee%20%28pERC%29/pcodr_perc_deliberative_frame.pdf. Accessed Jan 2020.
Health Quality Ontario. Health technology assessment methods and process guide. 2018. https://www.hqontario.ca/Portals/0/documents/evidence/reports/hta-methods-and-process-guide-en.pdf. Accessed Jan 2020.
The Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada 4th edition. 2017. https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada. Accessed Jan 2020.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. https://doi.org/10.1016/j.jval.2013.08.2291.
Mozygemba K, Hofmann B, Lysdahl K, Pfadenhauer L, Wilt G, Gerhardus A. Guidance to assess socio-cultural aspects. 2016. pp. 76–100.
Hofmann B. Toward a procedure for integrating moral issues in health technology assessment. Int J Technol Assess Health Care. 2005;21(3):312–8. https://doi.org/10.1017/s0266462305050415.
Hofmann B, Droste S, Oortwijn W, Cleemput I, Sacchini D. Harmonization of ethics in health technology assessment: a revision of the Socratic approach. Int J Technol Assess Health Care. 2014;30(1):3–9. https://doi.org/10.1017/s0266462313000688.
Matthias P, Reinhard B, Ansgar G, Bernhard G, Dagmar L, editors. Health Technology Assessment. Konzepte, Methoden, Praxis für Wissenschaft und Entscheidungsfindung. Berlin: Medizinisch Wissenschaftlicher Verlag, 2008. Berliner Schriftenreihe Gesundheitswissenschaften.
Vermeulen KM, Krabbe PFM. Value judgment of health interventions from different perspectives: arguments and criteria. Cost Eff Resour Alloc. 2018;16:16. https://doi.org/10.1186/s12962-018-0099-6.
Jain M NT, Jawla S, Rai N, Dev D, Weber S, Cook N. Heterogeneity in relative efficacy assessments (Rea) across European Hta bodies: opportunity for improving efficiency and speed of access to patients? ISPOR. 2015;18(7). https://doi.org/10.1016/j.jval.2015.09.1589.
The benefits of European cooperation. HTA & relative efficacy assessment. https://www.efpia.eu/about-medicines/use-of-medicines/hta-relative-efficacy-assessment/. Accessed Jul 2020.
Jonsson B. Bringing in health technology assessment and cost-effectiveness considerations at an early stage of drug development. MolOncol. 2015;9(5):1025–33. https://doi.org/10.1016/j.molonc.2014.10.009.
World Health Organization. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. 2001. http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf . Accessed Jun 2020.
Ghabri S, Mauskopf J. The use of budget impact analysis in the economic evaluation of new medicines in Australia, England, France and the United States: relationship to cost-effectiveness analysis and methodological challenges. Eur J Health Econ. 2018;19(2):173–5. https://doi.org/10.1007/s10198-017-0933-3.
Hampson G, Towse A, Henshall C. Assessing value, budget impact and affordability to inform discussions on access and reimbursement: principles and practice, with special reference to high cost technologies. 2017. https://www.ohe.org/publications/assessing-value-budget-impact-and-affordability-inform-discussions-access-and. Accessed Jun 2020.
Ackermann A-C: Budget impact in France: a new key to market access (17 June 2016).
European Patients' Academy. Ethical, social, and legal issues (ELSI) in HTA. 2016. https://www.eupati.eu/health-technology-assessment/ethical-social-and-legal-issues-elsi-in-hta/. Accessed Jun 2020.
Potter BK, Avard D, Graham ID, Entwistle VA, Caulfield TA, Chakraborty P, et al. Guidance for considering ethical, legal, and social issues in health technology assessment: application to genetic screening. Int J Technol Assess Health Care. 2008;24(4):412–22. https://doi.org/10.1017/s0266462308080549.
Shams Moattar A, Asghari F, Majdzadeh R. Do ethical considerations influence any in HTA reports? A review of reports. Med J Islam Repub Iran. 2016;30:362.
Mathes T, Willms G, Polus S, Stegbauer C, Messer M, Klingler C, et al. Health technology assessment of public health interventions: an analysis of characteristics and comparison of methods-study protocol. Syst Rev. 2018;7(1):79. https://doi.org/10.1186/s13643-018-0743-4.
Lehoux P, Williams-Jones B. Mapping the integration of social and ethical issues in health technology assessment. Int J Technol Assess Health Care. 2007;23(1):9–16. https://doi.org/10.1017/s0266462307051513.
Bowen S, Zwi AB. Pathways to “evidence-informed” policy and practice: a framework for action. PLoS Med. 2005;2(7):e166. https://doi.org/10.1371/journal.pmed.0020166.
Paula Corabian LT, Christa H. Evidence grading systems used in health technology assessment practice. 2018. https://www.ihe.ca/advanced-search/evidence-grading-systems-used-in-health-technology-assessment-practice. Accessed Jun 2020.
Author information
Authors and Affiliations
Contributions
YW conducted the study concept and design, literature search, acquisition of data, analysis and drafted the manuscript. TQ conducted the literature search, acquisition of data, analysis and revised the manuscript. JZ conducted the critical revision of the manuscript for important intellectual content. Professor CF and MT conducted the study concept and provided valuable supports to ensure the credibility and accuracy of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
All authors have no conflicts of interest to report in this work.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Qiu, T., Zhou, J. et al. Which Criteria are Considered and How are They Evaluated in Health Technology Assessments? A Review of Methodological Guidelines Used in Western and Asian Countries. Appl Health Econ Health Policy 19, 281–304 (2021). https://doi.org/10.1007/s40258-020-00634-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00634-0