Abstract
Background
Even though Insulin glargine (IGlar) has been available and used in other countries for more than a decade, it has not been adopted into Thai national formulary. This study aimed to evaluate the long-term cost effectiveness of IGlar versus neutral protamine Hagedorn (NPH) insulin in type 2 diabetes from the perspective of Thai Health Care System.
Methods
A validated computer simulation model (the IMS CORE Diabetes Model) was used to estimate the long-term projection of costs and clinical outcomes. The model was populated with published characteristics of Thai patients with type 2 diabetes. Baseline risk factors were obtained from Thai cohort studies, while relative risk reduction was derived from a meta-analysis study conducted by the Canadian Agency for Drugs and Technology in Health. Only direct costs were taken into account. Costs of diabetes management and complications were obtained from hospital databases in Thailand. Both costs and outcomes were discounted at 3 % per annum and presented in US dollars in terms of 2014 dollar value. Incremental cost-effectiveness ratio (ICER) was calculated. One-way and probabilistic sensitivity analyses were also performed.
Results
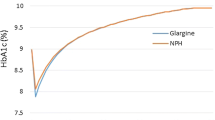
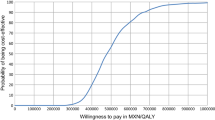
IGlar is associated with a slight gain in quality-adjusted life years (0.488 QALYs), an additional life expectancy (0.677 life years), and an incremental cost of THB119,543 (US$3522.19) compared with NPH insulin. The ICERs were THB244,915/QALY (US$7216.12/QALY) and THB176,525/life-year gained (LYG) (US$5201.09/LYG). The ICER was sensitive to discount rates and IGlar cost. At the acceptable willingness to pay of THB160,000/QALY (US$4714.20/QALY), the probability that IGlar was cost effective was less than 20 %.
Conclusions
Compared to treatment with NPH insulin, treatment with IGlar in type 2 diabetes patients who had uncontrolled blood glucose with oral anti-diabetic drugs did not represent good value for money at the acceptable threshold in Thailand.


Similar content being viewed by others
References
Bureau of Policy and Strategy. Statistical Thailand 2014. Office of the Permanent Secretary, Ministry of Public Health; 2014.
Aekplakorn W, Chariyalertsak S, Kessomboon S, Sangthong P, Inthawong R, Putwatana P, et al. Prevalence and management of diabetes and metabolic risk factors in Thai adults. Diabetes Care. 2011;34:1980–5.
Rawdaree P, Ngarmukos C, Deerochanawong C, Suwanwalaikorn S, Chetthakul T, Krittiyawong S, et al. Thailand diabetes registry (TDR) project: clinical status and long term vascular complications in diabetic patients. J Med Assoc Thai. 2006;89(Suppl 1):S1–9.
Pongcharoensuk P, Kongsaktrakool B, Tantivipanuwong S, Sema-ngern K, Chaiyakunapruk N. Costs of diabetes patients in Thai government hospitals. In: ISPOR 2nd Asia-Pacific Conference; Shanghai, China; 2006. p. 47.
Diabetes Association of Thailand, Endocrine Society of Thailand, Department of Medicine Services Ministry of Public Health, National Health Security Office. Thai Clinical Practice Guideline for Diabetes 2014. Bangkok; 2014.
Ngorsuraches S, Meng W, Kim BY, Kulsomboon V. Drug reimbursement decision making in Thailand, China, and South Korea. Value Health. 2012;15:S120–5.
Teerawattananon Y, Tritasavit N, Suchonwanich N, Kingkaew P. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwesen. 2014;108:397–404.
Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–74.
Jensen MG, Hansen M, Brock B, Rungby J. Differences between long-acting insulins for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2010;11:2027–35.
Waugh N, Cummins E, Royle P, Clar C, Marien M, Richter B, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14:1–268.
Rawdaree P, Sarinnapakorn V, Pattanaungkul S, Khovidhunkit W, Tannirandorn P, Peerapatdit T. A prospective, longitudinal, multicenter, observational study to assess insulin treatment patterns in diabetic patients in Thailand: Results from the TITAN study. J Med Assoc Thai. 2014;97:1140–50.
Tanvejsilp P, Ngorsuraches S. Defining the scope of health technology assessment and types of health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S10–6.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (type 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5–26.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(Suppl 1):S27–40.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53.
Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–8.
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–43.
World Health Organization. Life tables by country: Thailand. 2014 [October 15, 2014]; Available from: http://apps.who.int/gho/data/?theme=main&vid=61640.
Kosachunhanun N, Benjasuratwong Y, Mongkolsomlit S, Rawdaree P, Plengvidhya N, Leelawatana R, et al. Thailand Diabetes Registry project: glycemic control in Thai type 2 diabetes and its relation to hypoglycemic agent usage. J Med Assoc Thai. 2006;89(Supp 1):S66–71.
Krairittichai U, Potisat S, Jongsareejit A, Sattaputh C. Prevalence and risk factors of diabetic nephropathy among Thai patients with type2 diabetes mellitus. J Med Assoc Thai. 2011;94(Suppl2):S1–5.
Rangsin R, Tatsanavivat P, MedResNet. An assessment on quality of care among patients diagnosed with type 2 diabetes and hypertension visiting hospitals of Ministry of Public Health and Bangkok Metropolitan Administration in Thailand, 2012. Thailand: National Health Security Office (NHSO); 2012.
Nitiyanant W, Chetthakul T, Sang-A-kad P, Therakiatkumjorn C, Kunsuikmengrai K, Yeo JP. A survey study on diabetes management and complication status in primary care setting in Thailand. J Med Assoc Thai. 2007;90:65–71.
Supapluksakul S, Ruamviboonsuk P, Chaowakul W. The prevalence of diabetic retinopathy in Trang province determined by retinal photography and comprehensive eye examination. J Med Assoc Thai. 2008;91:716–22.
The Endocrine Society of Thailand. Diabetes Registry Project 2003. Health Systems Research Institute; 2004.
Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes. J Diabetes Complicat. 2014;28:124–9.
Thaneerat T, Tangwongchai S. Prevalence of depression, hemoglobin A1c level, and associated factors in outpatients with type 2 diabetes. Asian Biomed (Res Rev News). 2009;3:383–90.
Pornpinatepong S. Cost-effectiveness analysis of diabetic retinopathy screening in type 2 diabetes mellitus [Master Thesis]. Bangkok: Mahidol University; 2005.
Riewpaiboon A. Standard cost lists for health technology assessment. Health Intervention and Technology Assessment Program (HITAP); 2011.
National Statistical Office. Health. 2007 [15 October 2014]. Available from: http://web.nso.go.th/en/survey/bts/datafiles/560619_09_Health.pdf.
Thamarangsi T. The situation of alcohol beaverage consumption and impact in Thailand 2013. Center for Alcohol Studies, International Health Policy Program, Ministry of Public Health; 2013.
Teerawattananon Y, Mugford M, Tangcharoensathien V. Economic evaluation of palliative management versus peritoneal dialysis and hemodialysis for end-stage renal disease: evidence for coverage decisions in Thailand. Value Health. 2007;10:61–72.
Maharaj Nakorn Chiang Mai Hospital. Pharmacy and Healthcare Service Fees. Chiang Mai; 2014.
King Chulalongkorn Memorial Hospital. Pharmacy and Healthcare Service Fees. Bangkok; 2014.
National Health Security Office. Schedule of Health Benefits and Fees. Bangkok; 2014.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Mak. 2002;22:340–9.
Tamteerano Y, Khonputsa P, Chaikledkaew U, Teerawattananon Y, Lim S. Economic evaluation of HMG-CoA reductase inhibitors (statin) for primary prevention of cardiovascular diseases among Thai population (in Thai language). Bangkok: Health Intervention and Technology Assessment Program (HITAP), Ministry of Public Health; 2008.
Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637.
Australian Institute of Health and Welfare. The Burden of Disease and Injury in Australia; 2003.
Carrington AL, Mawdsley SK, Morley M, Kincey J, Boulton AJ. Psychological status of diabetic people with or without lower limb disability. Diabetes Res Clin Pract. 1996;32:19–25.
National Institute for Clinical Excellence. Guidance on the use of long-acting insulin analogues for the treatment of diabetes—insulin glargine 2002 Contract No.: 53.
Emerging risk factors collaboration, Seshasai SR, Kaptonge S, Thompson A, Di Angelantonio E, Gao P, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Association of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–51.
Canadian Agency for Drugs and Technologies in Health. Long-Acting Insulin Analogues for the Treatment of Diabetes Mellitus: Meta-analyses of Clinical Outcomes. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2008.
Chan SP, Ji LN, Nitiyanant W, Baik SH, Sheu WH. Hypoglycemic symptoms in patients with type 2 diabetes in Asia-Pacific: Real-life effectiveness and care patterns of diabetes management: The RECAP-DM study. Diabetes Res Clin Pract. 2010;89:e30–2.
Meeting summary of the National Drug Selection Working Group in Endocrine Number 4/2014 (Thai language). Food and Drug Administration, 3 October 2014.
Drug and Medical Supply Information Center. 2014 [Nov 2, 2014]; Available from: http://dmsic.moph.go.th.
Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S17–26.
Bureau of Trade and Economics Indices, Ministry of Commerce. CPI 2014 [October 12, 2014]; Available from: www.price.moc.go.th/price/cpi/index_new_e.asp.
Bank of Thailand. Foreign exchange rates 2015 [June 25, 2015]; Available from: https://www.bot.or.th/english/statistics/financialmarkets/exchangerate/_layouts/application/exchangerate/ExchangeRate.aspx.
Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai. 2014;97(Suppl 5):S50–8.
Thavorncharoensap M, Teerawattananon Y, Natanant S, Kulpeng W, Yothasamut J, Werayingyong P. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res. 2013;5:29–36.
Halpern EF, Weinstein MC, Hunink MG, Gazelle GS. Representing both first- and second-order uncertainties by Monte Carlo Simulation for groups of patients. Med Decis Mak. 2000;20:314–22.
Brandle M, Azoulay M, Greiner RA. Cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 2 diabetes mellitus, modeling the interaction between hypoglycemia and glycemic control in Switzerland. Int J Clin Pharmacol Ther. 2011;49:217–30.
Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and type 2 diabetes in Canada. Pharmacoeconomics. 2007;25:253–66.
McEwan P, Poole CD, Tetlow T, Holmes P, Currie CJ. Evaluation of the cost-effectiveness of insulin glargine versus NPH insulin for the treatment of type 2 diabetes in the UK. Curr Med Res Opin. 2007;23(Suppl 1):S21–31.
National Health Security Office. An assessment on quality of care among patients diagnosed with type 2 diabetes and hypertension visiting hospital of Ministry of Public Health and Bangkok Metropolitan Administry in Thailand. 2014; Available from: http://dmht.thaimedresnet.org/files_2558/57DMHT_2014_ALL_Ontour.pdf.
Health Technology Assessment Working Team. Health Technology Assessment in Thailand, 2nd ed. Nonthaburi; 2014.
Pemsuwan U, Chaiyakunapruk N, Dilokthornsakul P, Thavorn K, Saokaew S. Cost-effectiveness analysis of Insulin Glargine in the treatment of type 2 diabetes. National List of Essential Medicine; 2014.
Acknowledgments
This study was supported by Grants from the Subcommittees of the National List of Essential Medicine, Thailand. The authors gratefully acknowledge the support of the National Drug Selection Working Group in Endocrinology for their valuable comment on this study, and the IMS Health team for supporting the IMS CORE Diabetes Model.
Authors’ contributions
Unchalee Permsuwan designed the study; gathered the information for input parameters; ran the CDM; interpreted study findings; and prepared, edited, and approved the manuscript. Nathorn Chaiyakunapruk designed and conducted the study, interpreted the study results, and approved the final manuscript. Piyameth Dilokthornsakul and Surasak Saokaew were involved in the hospital database analyses, literature search for input parameters, and manuscript preparation and approval. Kednapa Thavorn performed the literature search for input parameters, developed a budget impact model, and prepared and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Unchalee Permsuwan, Nathorn Chaiyakunapruk, Piyameth Dilokthornsakul, Kednapa Thavorn, and Surasak Saokaew declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Permsuwan, U., Chaiyakunapruk, N., Dilokthornsakul, P. et al. Long-Term Cost-Effectiveness of Insulin Glargine Versus Neutral Protamine Hagedorn Insulin for Type 2 Diabetes in Thailand. Appl Health Econ Health Policy 14, 281–292 (2016). https://doi.org/10.1007/s40258-016-0228-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-016-0228-3