Abstract
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are the most severe cutaneous adverse reactions that are typically drug-induced in adults. Both SJS and TEN have high morbidity and mortality rates. SJS/TEN imposes clinical challenges for physicians managing patients suffering from this condition, both because it is rare and because it is a rapidly progressing systemic disease with severe cutaneous, mucosal, and systemic manifestations. Although many cases of SJS/TEN have been reported in the literature, there is no consensus regarding diagnostic criteria or treatment. Significant progress has been made in understanding its genetic predisposition and pathogenesis. This review is intended to provide physicians with a comprehensive but practical SJS/TEN roadmap to guide diagnosis and management. We review data on pathogenesis, reported precipitating factors, presentation, diagnosis, and management SJS/TEN focusing on what is new over the last 5 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a rare, T cell-mediated, severe cutaneous adverse reaction (SCAR) to medication. It is commonly associated with antibiotics and antiepileptics, though a wide array of drugs has been implicated. Mortality rates are high, and the sequelae can be debilitating. |
Because SJS/TEN is T cell mediated and is caused by small molecules perturbing the interaction between T cell receptors and peptides presented on MHC molecules, many of the genetic risk factors for SJS/TEN (including some that are clinically actionable) are related to antigen presentation, drug metabolism, and polymorphisms in the MHC complex. |
Supportive care remains the mainstay of all SJS/TEN treatment and a high-level evidence base is lacking for any single therapeutic intervention. TNF inhibitors (etanercept), cyclosporine, and combinations of different agents show some promise in unblinded randomized controlled trials and small observational studies. |
1 Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) comprise a spectrum of severe cutaneous adverse reactions (SCAR) with life-threatening acute effects and serious long-term sequelae. SJS/TEN is a type IV hypersensitivity reaction mediated by an immunologic response to a trigger, most commonly to drugs [1]. Infections may also cause the disease, and some cases remain idiopathic [2,3,4]. SJS/TEN is characterized by widespread skin and mucosal necrosis [5, 6]. The initial description was named after the physicians Stevens and Johnson in 1922, while the term toxic epidermal necrolysis was introduced by Lyell in 1956 [7]. Notably, the initial description of SJS was most likely post-infectious, and only one of Lyell’s handful of initial cases is likely true TEN. The 1993 formal consensus definition classifies cases according to the body surface area (BSA) detached. SJS was defined as < 10% BSA detached, SJS/TEN overlap with 10–30% of BSA detached, and TEN with >30% BSA detached [7]. Although approximately 80% of SJS/TEN cases in adults are medication-associated, in children and young adults, two mimickers, erythema multiforme (EM), and reactive infectious mucocutaneous eruption (RIME; previously known as mycoplasma-induced rash and mucositis or MIRM) are prevalent and should be considered [8]. Immunotherapy used in cancer, like immune checkpoint inhibitors, also are associated with SCAR-like reactions, including SJS/TEN-like reactions, that may be difficult to distinguish from “true” SJS/TEN and often show histopathology more consistent with bullous lichenoid drug eruption or bullous pemphigoid [9,10,11]. With continued research into immunopathogenesis, it is likely in the future that a more precise classification will be based on molecular markers and histopathological findings.
SJS/TEN has a reported incidence of 1–5 cases per 1,000,000 individuals annually and has a higher incidence in adults than pediatric patients, likely due to increased exposure to potential triggers [6, 12]. However, because SJS shares International Classification of Diseases, Tenth Revision (ICD-10) codes with erythema multiforme, incidence may be overestimated. Furthermore, 36–72% of patients initially diagnosed with SJS/TEN end up having their diagnosis reclassified into a different disease, also contributing to an overestimation of SJS/TEN incidence [13]. Incidence of SJS/TEN also varies by country, partly due to (1) differences in genetic background and (2) differing prescribing patterns [14]. For example, the Han Chinese have a high carrier rate of HLA-B*15:02, an allele strongly associated with carbamazepine-induced SJS/TEN. This association is one explanation for why carbamazepine-induced SJS/TEN has a higher incidence in Southeast Asia than in Europe [15]. Human immunodeficiency virus (HIV) and tuberculosis (TB) endemic locales, like several countries in Africa, also often have higher rates of SJS/TEN owing to an increased use of anti-HIV and anti-TB medications that are high risk for SJS/TEN [16]. Table 1 summarizes known genetic and ethnic associations with SJS/TEN.
Because it causes widespread detachment of skin and mucosal surfaces, SJS/TEN can cause severe complications, including superimposed infection, sepsis, organ dysfunction, and death. Reported mortality rates are as high as 34–50%, with mortality correlating with BSA involvement [1, 5, 12, 17]. In addition, patients with SJS/TEN have a mean loss of life expectancy of approximately 9 years [18]. In addition to morbidity and mortality, expectancy, cost, and readmissions are important to consider in SJS/TEN patients [5, 19]. This review aims to provide an overview and update on the pathogenesis, precipitating factors, presentation, diagnosis, and management of SJS/TEN.
2 Pathogenesis
SJS/TEN is a severe T cell mediated type IV (delayed) hypersensitivity reaction. Over 80% of cases are associated with medication exposure, particularly antimicrobials (sulfa antibiotics), antiepileptics, allopurinol, and nonsteroidal anti-inflammatory drugs (NSAIDs) [20]. More than 300 different drugs and supplements have been implicated [21]. Most cases occur 4–28 days after initial exposure to the drug, though case reports for delayed-onset (6 months) SJS/TEN to lamotrigine and rapid-onset SJS/TEN to acetaminophen and penicillin (3 days) have been reported [22,23,24]. With rapid onset SJS/TEN, an infectious etiology with protopathic introduction of drugs following first onset of SJS/TEN symptoms should be considered.
The current understanding of SJS/TEN pathogenesis is summarized in Fig. 1. The key event is a tripartite interaction between a peptide presented by a major histocompatibility complex (MHC) on an antigen-presenting cell (APC) and a T cell receptor (TCR) expressed on a CD8+ (cytotoxic) T cell. These drug-reactive T cells may derive from skin-resident memory T cells (TRM) and/or circulating CD8+ T cells [25]. There are many proposed mechanisms by which a drug or reactive metabolite alters a self-protein and activate T cells. In the hapten model and the pro-hapten model, the drug or a metabolite, respectively, covalently modifies the presented peptide, making a previously nonimmunogenic peptide immunogenic. In the pharmacological interaction with immune receptors (p-I) model, the drug noncovalently inserts in the immunological synapse, altering activation. In the altered TCR repertoire or altered peptide model, the drug or its metabolite binds directly to the HLA complex or the TCR (respectively), altering either the peptide repertoire or the confirmation of TCR. Examples of each of these mechanisms have been reported in literature [26].
This figure describes the various pathways that lead to keratinocyte apoptosis and necroptosis, including interactions between antigen-presenting cells (APC), T cells, natural killer cells, monocytes, and keratinocytes. Natural killer (NK) cells can trigger keratinocyte apoptosis through interaction of CD94/NKG2C with HLA-E on keratinocytes and are the likely source of granulysin. APC present a peptide on a major histocompatibility complex (MHC) to a T cell receptor (TCR) expressed on a CD8+ (cytotoxic) T cell. The activated CD8+ T cells then trigger downstream cytokine/chemokine production and epidermal keratinocyte apoptosis through the Fas/Fas ligand (FasL) and the TCR/human leukocyte antigen (HLA) pathways. Additionally, drug-activated monocytes may trigger necroptosis of keratinocytes via Annexin A1 binding to formyl peptide receptor 1 (FPR1). The figure also outlines targets of treatment options including intravenous immunoglobulin, etanercept, and tumor necrosis factor. Abbreviations: TNF = tumor necrosis factor, HMGB1 = high mobility group box 1, TRAIL = TNF-related apoptosis-inducing ligand, FasL = Fas ligand, IL = interleukin, HLA = human leukocyte antigen, CD = cluster of differentiation, NKG2C = natural killer gene 2C, TCR = T cell receptor, MHC = major histocompatibility complex, TRM = skin-resident memory T cells, CYP450 = cytochrome P450. Created with biorender.com
After the initial interaction, the activated CD8+ T cells trigger downstream cytokine/chemokine production and epidermal keratinocyte apoptosis through the Fas/Fas ligand (FasL) pathway and TCR/HLA pathway. Keratinocytes likely also trigger apoptosis of other keratinocytes through Fas/FasL signaling, though keratinocyte expression of FasL is controversial [27, 28]. Natural killer (NK) cells can trigger keratinocyte apoptosis through interaction of CD94/NKG2C with HLA-E on keratinocytes and are likely a source of the apoptotic mediator granulysin [29]. Additionally, drug-activated monocytes may trigger necroptosis of keratinocytes via Annexin A1 binding to formyl peptide receptor 1 (FPR1) [30]. Blister fluid collected from SJS/TEN patients has been shown to predominantly contain clonally-expanded CD8+ T cells, in addition to NK cells, monocytes, macrophages, and other immune cells; the fluid also contains several apoptotic mediators, such as TRAIL, perforin, granzyme, TNF-α, soluble FasL, and granulysin [27, 31,32,33,34,35,36].
Recent work has also suggested a larger role for innate immunity in SJS/TEN pathogenesis, beyond the involvement of NK cells, monocytes, and macrophages. A recent report showed that neutrophils abet inflammation in the early stages of SJS/TEN, by undergoing NETosis and releasing mediators causing necroptosis of keratinocytes [37]. Polymorphisms in toll-like receptor 3 (TLR3), prostaglandin-E receptor 3 (PTGER3), and IKAROS family zinc finger 1 (IKZF1) were associated with cold-medicine-induced ocular SJS/TEN [38]. Chronic-stage ocular SJS/TEN has also shown upregulation in several mediators, including IL-8, IL-6, and interferon-gamma [39].
Because of the importance of MHC to the pathogenesis of SJS/TEN and other drug hypersensitivity reactions, many of the genetic variants most closely linked to the condition are HLA alleles. In particular, HLA-B*15:02 is strongly associated with aromatic anticonvulsant-associated SJS/TEN, with reported odds ratios (OR) for carbamazepine ranging from 17 to 1357 in white and Asian populations [40]. HLA-B genotyping can be considered before prescribing fosphenytoin, phenytoin, oxcarbazepine, and carbamazepine; however, the association has negative predictive value (NPV) significantly less than 100%, particularly in non-East-Asian populations where the association is not as strong [41]. This is in contrast to the absence of the HLA-B*57:01 allele, which has an NPV of 100% for abacavir hypersensitivity (a reaction distinct from SJS/TEN, though illustrative as an example); this reaction previously occurred in approximately 5% of patients receiving the drug, but HLA-B*57:01 screening has eliminated it [42,43,44]. Similarly, implementation of HLA-B*15:02 screening in Taiwan decreased incidence of carbamazepine-induced SJS/TEN to 0 [45].
A few other genetic risk factors shed light on the pathogenesis of SJS/TEN—polymorphisms in cytochrome P450 and other genes required for drug metabolism, and polymorphisms in the antigen presentation pathway. For example, CYP2C9 and CYP2C19 variants (in particular CYP2C9 poor metabolizer genotypes), are associated with phenytoin-induced SJS/TEN even in the absence of HLA-B*1502 [46, 47]. Another study showed that polymorphisms in the proteasome pathway (required for trimming peptides for presentation on MHC) are associated with SJS/TEN [26, 48]. However, no combination of these variants has 100% NPV, and only a small portion carrying genetic risk factors (2–8%) will develop SJS/TEN. Therefore, further research is needed to better understand these risk factors and effectively use them in a clinical setting.
3 Clinical Presentation
SJS/TEN typically begins within 4 weeks of drug exposure. First symptoms are typically non-specific and precede cutaneous symptoms by a few days in up to one third of cases. Early symptoms may include headache, rhinitis, cough, sore throat, or myalgias. At this stage, it is important to have a broad differential, as patients are often misdiagnosed initially. In other cases, mucosal or cutaneous symptoms may be a part of the initial presentation [49, 50].
Over the next few days, cutaneous symptoms begin rapidly. Macular atypical targetoid lesions appear and often become confluent [51]. These progress to vesicles and bullae, eventually developing into the hallmark full-thickness necrosis, detachment, and skin sloughing [49, 51]. The affected skin is often severely painful [51]. The epithelium of mucosal surfaces, such as the lips, mouth, oropharynx, respiratory tract, gastrointestinal tract, and genitalia, may become necrotic, leading to erosions, ulcerations, and detachment [51, 52]. Ocular involvement is frequent in acute SJS/TEN and may not become apparent until the more chronic stages [53]. Ocular complications of SJS/TEN are broad including, chronic dry eyes, corneal inflammation, trichiasis, symblepharon, and lid margin keratinization [53]. Dysosmia and dysgeusia have also been reported as complications of mucosal involvement in SJS/TEN [54]. Examples of cutaneous, ocular, genital, and oral involvement are shown in Fig. 2.
Systemic organ involvement can occur in SJS/TEN through a variety of mechanisms. Epidermal barrier breakdown can lead to homeostatic dysfunction, electrolyte derangement, hypothermia, dehydration, and/or sepsis. Organs with an epithelial lining can be directly affected, leading to respiratory distress syndrome, colitis, pancreatic injury, liver dysfunction, and other complications [27, 51, 55]. Cross-reactivity to bone marrow antigens may cause pancytopenia in some patients [56].
4 Clinical Assessment
Red flags on initial examination include skin pain, prodromal symptoms and mucositis in relation to a rapidly developing extensive rash. On presentation, a thorough skin examination should be conducted to evaluate the extent of cutaneous involvement, along with examination by ophthalmology, urology, and gynecology specialists for mucosal involvement. Skin, ocular, and oral examinations should be conducted daily. Patients should also be closely examined and monitored for systemic involvement [57]. Laboratory assessment including complete blood count, a comprehensive metabolic panel to assess electrolyte status in the setting of significant insensible losses, and baseline arterial blood gas to determine respiratory status are essential on initial evaluation. Additionally, electrolytes, glucose, and fluid balance should be monitored daily during admission and managed appropriately [58]. Imaging should be obtained if clinically indicated. Given the high prevalence of oral and mucosal involvement, it is also critical to evaluate if there is a need for orotracheal intubation [59].
4.1 Diagnosis
There are no standard diagnostic criteria for SJS/TEN, though the presence of macular targetoid lesions, involvement of two mucous membranes, recent drug exposure, and corresponding histopathology are all suggestive. As defined in the introduction, the clinical differentiation between SJS and TEN is based on body surface area involvement specifically of detached or detachable skin. Other lesions that may appear on intact skin (and thus do not count towards BSA involvement), include morbilliform rash, erythematous macules or patches, purpura, and targetoid lesions. SJS is when < 10% BSA is affected, SJS-TEN overlap is 10%–30% BSA, and TEN is > 30% BSA [60].
Clinical features of SJS/TEN can mimic other dermatologic diseases, including erythema multiforme, reactive infectious mucocutaneous eruption, generalized bullous fixed drug eruption, staphylococcal scalded skin syndrome, pemphigus vulgaris, and acute graft versus host disease, among others (Table 2). It is important to differentiate between these conditions, as they have different treatments and prognoses. In particular, note that Nikolsky sign is not pathognomonic for SJS/TEN alone, as it can be present in multiple diseases [61].
Histologic features of SJS/TEN include full thickness epidermal necrosis, keratinocyte apoptosis, basal vacuolar change, subepidermal bullae, subepidermal clefting, and mild T cell infiltrate [62,63,64]. Drug-induced SJS/TEN skin biopsies (versus infection or immunization-induced) may have a dermal infiltrate with a high number or eosinophils or neutrophils [64]. Histologic findings may also be indicative of severity of disease or worse prognosis; for example, skin biopsies from patients with TEN (versus SJS) may have a more significant dense dermal mononuclear infiltrate [65]. New research has supported that ex vivo confocal laser scanning microscopy may serve as a safe, rapid, non-invasive alternative to skin biopsy, though this is not yet widely available [66].
Historically, erythema multiforme major (EMM) has been particularly difficult to differentiate from SJS/TEN [67]. There are various diagnostic tests that can assist. EM generally is typically characteristic by the appearance of both macular and papular typical targetoid lesions (with three zones of color, as opposed to atypical targetoid lesions which have two) and papular atypical targetoid lesions, all of which are generally absent in SJS/TEN which only has macular atypical targetoid lesions [68]. Immunohistochemistry for cytotoxic molecules (such as granulysin, perforin, and granzyme B), CD4 and Treg can help differentiate SJS/TEN from EMM [69]. Demographics (with EMM skewing younger) and risk factors (drug exposure for SJS/TEN versus respiratory infection for EMM) should also be considered [70]. Direct immunofluorescence (DIF) and enzyme-linked immunosorbent assay (ELISA) are important to rule out autoimmune bullous disease, severe cases of which can mimic SJS/TEN [63, 71, 72].
Much recent research has focused on biomarkers for SJS/TEN diagnosis and prognostication. Granulysin is a cytotoxic mediator involved in keratinocyte death and is highly expressed in SJS/TEN blister fluid and serum [31, 73, 74]. Blister fluid and serum granulysin concentration have been found to have a linear relationship with BSA involvement [31, 75]. Serum granulysin has a sensitivity of 80% and specificity of 95.7% for early diagnosis of SJS/TEN in patients with nonspecific drug rash, and a rapid immunochromatographic test strip for granulysin has been developed, though is not yet widely available [76]. Serum granulysin may also be used as a predictor of SJS/TEN development 2–4 days prior to skin detachment or development of mucosal lesions (p < 0.010) [73]. It is, however, important to note that granulysin has also been detected on histopathology in the inflammatory infiltrate patients with other cutaneous adverse drug reactions including drug reaction with eosinophilia and systemic symptoms (DRESS) and fixed drug eruption [77]. Furthermore, granulysin has not been validated in large cohorts and thus has not been widely adopted in clinical settings. Other biomarkers (IL-15, HMGB1, IL-13, etc.) have also been studied, but none are currently considered standard-of-care [78,79,80].
HLA testing has a role in risk stratification of patient populations as an option prior to starting SJS/TEN culprit drugs. Several studies have estimated positive predictive value (PPV) and NPV of HLA testing for patients who develop SJS/TEN, which tend to differ by ethnic group [81]. Understanding HLA associations with drugs implicated in the development of SJS/TEN can help with avoidance of a particular drug and/or knowledge to prevent re-exposure if the patient is an SJS/TEN survivor.
4.2 Determination of Causality
Determining causality is critical for prompt discontinuation of the culprit drug and strict avoidance of the culprit drug and potentially cross-reactive drugs in the future [82]. Causality is primarily determined through careful history-taking and construction of a drug timeline, including prescription drugs, over-the-counter medications, and supplements. Determining when each drug was started, stopped, held, or had a dose change is critical. It is also important to consider whether the patient has a history of cutaneous adverse reactions to medications or has underlying comorbidities, such as cancer or HIV.
Multiple algorithms have been developed to assist with the determination of drug causality, including the algorithm of drug causality for epidermal necrolysis (ALDEN), the Liverpool Causality Assessment Tool, and the Naranjo scale [82, 83]. The ALDEN score is considered the gold-standard; it ranges from −12 to 10, and is determined by six factors—the time between drug initiation and onset of symptoms, the half-life of the drug, whether the patient had previously taken the drug (prechallenge), whether the drug was continued beyond the progression phase of the disease (dechallenge), drug notoriety, and other possible alternatives [84]. Comparing patient history with ALDEN scores has revealed correlations with other medical conditions that potentially increase the risk of SJS/TEN, such as psoriasis, history of drug reactions or allergies, systemic lupus erythematosus, malignancy, and diabetes mellitus [83]. In contrast, the Naranjo score ranges from −4 to 13, and consists of ten questions. Unlike ALDEN, the Naranjo score accounts for confirmation by objective evidence, detection of the drug in blood, and whether the reaction reappeared with placebo or readministration [85]. The Liverpool algorithm was developed from the Naranjo scale, due to several questions in the latter often having answers of “unknown” or “unable to assess” [86].
Determining drug causality after the fact is also an important area of research. Post-hoc testing methods include ex vivo/in vitro methods, such as lymphocyte transformation testing (LTT), ELIspot, and cytokine release assays, and in vivo methods, such as epicutaneous patch testing (PT) [87]. Intradermal (“prick”) testing (IDT) has not been recommended owing to the theoretical risk of reproducing the original reaction [88]. However, skin testing (both PT and IDT) for SJS/TEN has low sensitivity. In a systematic review, reported positivity rates for PT ranged from 13 to 33% (which increases to 54–77% for focused testing to suspected medication) [89, 90]. This varies widely by drug—allopurinol (and its metabolite, oxypurinol, thought to be causative) is usually negative, with a sensitivity of 0%, while antiepileptics and antibiotics are positive more frequently [89, 91]. In general, patch testing seems to have much greater utility in other types of severe cutaneous adverse reactions, like DRESS, acute generalized exanthematous pustulosis (AGEP), and morbilliform drug eruption (MDE), than SJS/TEN [92, 93]. Partly because of this low sensitivity, patch testing has not been widely adopted, and more large-scale studies are needed.
Several culture-based assays, in which patients’ peripheral blood mononuclear cells (PBMC) are co-cultured with suspected medication, have also been developed. In LTT, cultured cells are assayed for proliferation. Other assays measure cytokines in cell culture, detecting intracellular apoptotic mediators by flow cytometry, or detect the release of mediators, such as granzyme from individual cells via ELIspot [88, 94]. While LTT alone has low sensitivity, combining multiple assays together can increase the sensitivity substantially (in one study up to 80%) [94,95,96]. Of note, these assays are not commercially available and as a result, their use is limited.
4.3 Severity Assessment
Given that SJS/TEN has a high mortality rate, estimated to be between 34 and 50% globally, the most critical clinical assessments are severity and mortality risk [97, 98]. Severity and mortality risk of SJS/TEN can be estimated with several validated tools, including the Score of Toxic Epidermal Necrolysis (SCORTEN); the revision of SCORTEN (Re-SCORTEN); the age, bicarbonate, cancer, dialysis, and 10% body surface area risk model (ABCD-10); and the clinical risk score for toxic epidermal necrolysis (CRISTEN).
SCORTEN is well-established as a method to determine mortality risk [12]. SCORTEN utilizes the following seven clinical indicators: age > 40 years, active cancer, heart rate > 120 beats per min, serum blood urea nitrogen > 28 mg/dL, detached or compromised body surface > 10%, serum bicarbonate < 20 mmol/L, and serum glucose > 250 mg/dL [7, 99, 100]. SCORTEN should be calculated on day 1 and 3 of hospitalization and is used to score severity and estimate mortality by using the above variables to calculate probability of death [7].
Koh et al. proposed a revision of SCORTEN (Re-SCORTEN) for mortality prognostication, adding the red blood cell distribution width to hemoglobin ratio (RHR), which can be determined from a basic complete blood count. The authors incorporated RHR into SCORTEN by adding a value of 2 for patients with RHR > 1.19, which led to significantly increased prognostic accuracy [101].
ABCD-10 is a risk prediction model for severity and mortality that uses five indicators (age > 50 years, body surface area > 10%, serum bicarbonate < 20 mmol/L, active cancer, and prior dialysis) [100, 102]. This differs from SCORTEN by increasing the weight of cancer on prognosis and by including history of prior dialysis instead of only current kidney function [102]. However, this model has been found to be inferior to SCORTEN at mortality prediction [103].
CRISTEN is a novel risk prediction model of SJS/TEN to predict severity and mortality that uses ten clinical parameters (without the immediate need for laboratory values): age > 65 years; epidermal detachment > 10% of BSA; active cancer; diabetes mellitus on treatment with medication; chronic kidney disease; bacterial infection including pneumonia, sepsis, or urinary tract infection; cardiac disease including hypertension under treatment; antibiotics in the list of culprit drugs; mucosal damage affecting ocular, buccal, and genital mucosa; and recent systemic corticosteroid therapy before the onset of SJS/TEN [104]. The benefit of CRISTEN is that it does not require laboratory testing prior to prediction of mortality, which may improve versatility and promptness; rather, it uses clinical features. The validation study did have a lower area under the curve than the creation study (p > 0.05); however, it may be more beneficial to utilize this scoring system as an adjunct to SCORTEN for early prognostication [104].
Many SJS/TEN scoring systems are prognostic models and do not allow for dynamic assessment or incorporate cutaneous morphology traits. A recent Delphi consensus exercise redefined morphology and distribution terminology for TEN and reinforced the need for developing a skin-directed and morphologically based SJS/TEN scoring system [105]. There are additional scoring assessments that are available, such as time to partial re-epithelialization, time to complete re-epithelialization, and BSA involved, among others; however, use should be discouraged until these are validated [100].
Beyond validated scores, a few laboratory values have emerged as potential markers of severity. These include lactate dehydrogenase, creatine kinase, granulysin, and interleukin-15, all four of which correlate directly with BSA involvement [73, 75, 106, 107]. There is also evidence that a positive anti-SS-A serology ay predict worse outcomes and therefore may prompt more aggressive treatment [108]. While these studies are limited, it is a promising opportunity for further research and may become useful for clinical practice to predict severity early in the disease process.
5 Treatments
5.1 Supportive Care
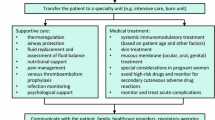
Treatment for SJS/TEN is complex, and there are no standardized guidelines for treatment currently. Our inability to accurately measure severity and appropriate outcomes is a major hindrance to determining standard of care. Withdrawal of the offending drug and all nonessential medications is critical, followed by hospitalization and supportive care. Furthermore, owing to the potential of multi-organ involvement, multidisciplinary care is often required [109,110,111,112].
Depending on the severity, the patient may be transferred to a burn center or intensive care unit [60]. Aggressive supportive care is the mainstay of initial management, and should include wound care, oral care, ocular care, genitourinary care, pain management, airway management, fluid and electrolyte management, stress ulcer prophylaxis, nutrition management, and deep vein thrombosis prophylaxis [58].
A particularly important consideration in SJS/TEN is the prevention of sepsis, which is the major cause of mortality in these patients [113]. Maintenance of an aseptic environment is critical and careful septic handling is required. Some advocate aggressive surgical debridement, particularly for TEN, to remove necrotic skin as a potential source of infection, while others advocate conservative management with anti-shear measures, leaving devitalized skin intact to function as a natural bandage; there is currently no consensus, and both approaches have shown equivalent re-epithelialization rates [114]. Close monitoring of body temperature and hemodynamic status, along with frequent culture of skin, urine, and blood specimens for bacteria and fungi is warranted. While prophylactic antibiotics are not recommended as part of usual supportive care, prompt use of antibiotics in the setting of clinical infection is likely critical.
5.2 Systemic Treatment
There is no high-level evidence for the treatment of SJS/TEN; the available studies include two open randomized controlled trials (RCT) and several small observational studies, case series, or retrospective reviews. Medications that have been reported to have some benefit are summarized in Table 3.
Cyclosporine, used on the basis of its T cell-specific mechanism, shows promise as an immunomodulatory medication that in small observational studies has had positive impact on hospital stay and progression of skin detachments in SJS/TEN patients [49, 115,116,117]. However, cyclosporine can be nephrotoxic and is avoided in patients who have a kidney injury, as kidney function is a critical component of SCORTEN and ABCD-10 prognoses.
Oral and intravenous corticosteroids are often used. Studies have suggested that prompt initiation of high dose corticosteroids within 1–2 days of symptoms onset leads to improved outcomes [118, 119]. However, other evidence suggests that the use of corticosteroids is associated with increased risk of infection [120]. Intravenous immunoglobulin (IVIG), which likely works via inhibition of the Fas receptor, has been used both alone and in combination with corticosteroids [28]. A network meta-analysis published in 2021 showed that corticosteroid/IVIG combination therapy was the only with a mortality benefit compared to control [121]. However, many studies suggest limited benefit to IVIG, and it could be harmful in those with renal impairment [122,123,124]. It is likely for such treatment including IVIG and corticosteroids that the time window to initiation to achieve a beneficial effect is very short to be feasible in clinical practice.
Biologic TNF-α inhibitors, such as etanercept, have been shown to be an effective treatment with minimal side effects [74, 125,126,127,128,129]. In a 2022 Cochrane review, etanercept twice weekly until healing was the only treatment that reached low-certainty evidence, possibly offering a superior mortality benefit to corticosteroids [130]. Patients treated with TNF-α inhibitors have achieved complete skin re-epithelialization and in a shorter prior of time than patients treated with other treatments such as corticosteroids [49]. A randomized, controlled open-labelled trial comparing intravenous corticosteroids to etanercept showed a significant decrease in time to re-epithelialization [74]. Interestingly the combination of etanercept and other treatments may show benefit—a multicenter retrospective study showed that the combination of etanercept and corticosteroids showed improved mortality rates compared with the combination of corticosteroids/IVIG and corticosteroids alone [131].
Special populations, such as pregnant patients and children who may require additional considerations and treatment modifications. For example, when treating a pregnant woman with SJS/TEN, careful consideration regarding fetal status, delivery method, and whether the disease has affected the fetus must be considered [49].
6 Chronic Complications and Care of the SJS/TEN Survivor
Patients with SJS/TEN may suffer from numerous chronic complications (Table 4). As during the acute illness, multidisciplinary follow up care is also recommended upon discharge to monitor for sequelae that impact quality of life. Dental/oral, ocular, genital, and psychological sequelae are common [132].
Survivors of SJS/TEN experience tremendous psychosocial effects that are often underreported. It is important to screen patients with SJS/TEN during hospitalization and in the follow-up period for psychiatric illnesses [133]. Survivors report high rates of post-traumatic stress disorder, depression, and anxiety [134, 135]. Physicians may utilize validated questionaries to screen survivors for psychiatric status, such as Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder-7 (GAD-7), and Primary Care Post-Traumatic Stress Disorder Screen (PC-PTSD) [134]. Given the complex nature of chronic complications, particularly psychosocial, there are many support groups for survivors located internationally (Table 5).
In addition to an increased rate of psychosocial effects, SJS/TEN survivors have an estimated reduced life expectancy of about 9 years and an increased risk of ensuing higher healthcare-related costs [136, 137]. The decreased life expectancy may be owing to a reduced and delayed usage of high-risk drugs that may be associated with SJS/TEN. Often, survivors are left avoiding multiple medications if the SJS/TEN trigger was not clearly identified. In situations where use of a medication is necessary and alternatives are not available, there can be shared decision making with the patient to consider a drug challenge test in unique scenarios [138]. Genetic screening can be done prior to use, but this may lead to a delay in treatment [18, 136].
Professor Jean‐Claude Roujeau, one of the most important leaders of SJS/TEN, suggested three key objectives when supporting SJS/TEN survivors: (1) carefully listen to patients concerns and collaborate with them to treat psychosocial distress, (2) advance clinical and basic research to better understand SJS/TEN long-term sequalae, and (3) ensure patients have equitable access to health care, and consider if patients can earn compensation in some capacity. Given the high mortality rate of patients with SJS/TEN, Professor Jean‐Claude Roujeau advocated for affected patients to be termed true “victims” [139].
7 Conclusion and Future Directions
This review aims to provide an overview and update on the pathogenesis, reported precipitating factors, genetic risk factors, presentation, diagnosis, and management of SJS/TEN. Important areas of further study include continued elucidation of immunopathogenesis and genetic associations, investigation into potential biomarkers to aid in diagnosis and prognostication, and standardization of optimal treatment. These advances will allow for superior preventative screening, diagnosis, and management of SJS/TEN.
References
Wasuwanich P, So JM, Chakrala TS, Chen J, Motaparthi K. Epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the United States and factors predictive of outcome. JAAD Int. 2023;13:17–25.
Monnet P, Rodriguez C, Gaudin O, Cirotteau P, Papouin B, Dereure O, et al. Towards a better understanding of adult idiopathic epidermal necrolysis: a retrospective study of 19 cases. J Eur Acad Dermatol Venereol. 2021;35(7):1569–76.
Chaby G, Ingen-Housz-Oro S, De Prost N, Wolkenstein P, Chosidow O, Fardet L. Idiopathic Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and patients’ characteristics. J Am Acad Dermatol. 2019;80(5):1453–5.
Welfringer-Morin A, Bataille P, Drummond D, Bellon N, Ingen-Housz-Oro S, Bonigen J, et al. Comparison of idiopathic and drug-induced epidermal necrolysis in children. Br J Dermatol. 2023;189(5):631–3.
Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States Adults. J Invest Dermatol. 2016;136(7):1387–97.
Liotti L, Caimmi S, Bottau P, Bernardini R, Cardinale F, Saretta F, et al. Clinical features, outcomes and treatment in children with drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis. Acta Biomed. 2019;90(3-S):52–60.
Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–6.
Pan CX, Hussain SH. Recurrent reactive infectious mucocutaneous eruption: a retrospective cohort study. J Am Acad Dermatol. 2023;89(2):361–4.
Molina GE, Yu Z, Foreman RK, Reynolds KL, Chen ST. Generalized bullous mucocutaneous eruption mimicking Stevens-Johnson syndrome in the setting of immune checkpoint inhibition: a multicenter case series. J Am Acad Dermatol. 2020;83(5):1475–7.
Reschke R, Mockenhaupt M, Simon JC, Ziemer M. Severe bullous skin eruptions on checkpoint inhibitor therapy—in most cases severe bullous lichenoid drug eruptions. J Dtsch Dermatol Ges. 2019;17(9):942–8.
Satoh TK, Neulinger MM, Stadler PC, Aoki R, French LE. Immune checkpoint inhibitor-induced epidermal necrolysis: a narrative review evaluating demographics, clinical features, and culprit medications. J Dermatol. 2024;51(1):3–11.
Marks ME, Botta RK, Abe R, Beachkofsky TM, Boothman I, Carleton BC, et al. Updates in SJS/TEN: collaboration, innovation, and community. Front Med (Lausanne). 2023;10:1213889.
Bettuzzi T, Hoisnard L, Beytout Q, Ingen-Housz-Oro S, Sbidian E. Validation of an algorithm to identify epidermal necrolysis on a medico-administrative database. J Invest Dermatol. 2023. https://doi.org/10.1016/j.jid.2023.09.274.
Roberson ML. Precision in language regarding geographic region of origin in severe cutaneous adverse drug reaction research. JAMA Dermatol. 2024;160(5):534.
Ferrell PB Jr, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9(10):1543–6.
Peter J, Choshi P, Lehloenya RJ. Drug hypersensitivity in HIV infection. Curr Opin Allergy Clin Immunol. 2019;19(4):272–82.
Alajaji A, Chandra Shekaran J, Mohammed Aldhabbah O, Alhindi HA, Almazyad NS, Aljutayli ZA, et al. Toxic Epidermal Necrolysis (TEN)/Stevens-Johnson Syndrome (SJS) Epidemiology and Mortality Rate at King Fahad Specialist Hospital (KFSH) in Qassim Region of Saudi Arabia: a retrospective study. Dermatol Res Pract. 2020;2020:7524726.
Chiu YM, Chiu HY. Lifetime risk, life expectancy, loss-of-life expectancy and lifetime healthcare expenditure for Stevens-Johnson syndrome/toxic epidermal necrolysis in Taiwan: follow-up of a nationwide cohort from 2008 to 2019. Br J Dermatol. 2023;189(5):553–60.
Guzman AK, Zhang M, Kwatra SG, Kaffenberger BH. Predictors of 30-day readmission in Stevens-Johnson syndrome and toxic epidermal necrolysis: a cross-sectional database study. J Am Acad Dermatol. 2020;82(2):303–10.
Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, et al. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138(11):2315–21.
Wang L, Varghese S, Bassir F, Lo YC, Ortega CA, Shah S, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review of PubMed/MEDLINE case reports from 1980 to 2020. Front Med (Lausanne). 2022;9: 949520.
Jha KK, Chaudhary DP, Rijal T, Dahal S. Delayed Stevens-Johnson syndrome secondary to the use of lamotrigine in bipolar mood disorder. Indian J Psychol Med. 2017;39(2):209–12.
Kim EJ, Lim H, Park SY, Kim S, Yoon SY, Bae YJ, et al. Rapid onset of Stevens-Johnson syndrome and toxic epidermal necrolysis after ingestion of acetaminophen. Asia Pac Allergy. 2014;4(1):68–72.
Poulsen VO, Nielsen J, Poulsen TD. Rapidly developing toxic epidermal necrolysis. Case Rep Emerg Med. 2013;2013: 985951.
Schunkert EM, Shah PN, Divito SJ. Skin resident memory T cells may play critical role in delayed-type drug hypersensitivity reactions. Front Immunol. 2021;12: 654190.
Cheng L. Current pharmacogenetic perspective on Stevens-Johnson Syndrome and toxic epidermal necrolysis. Front Pharmacol. 2021;12: 588063.
Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. 2003;162(5):1515–20.
Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282(5388):490–3.
Morel E, Escamochero S, Cabanas R, Diaz R, Fiandor A, Bellon T. CD94/NKG2C is a killer effector molecule in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol. 2010;125(3):703–10 (10 e1-10 e8).
Saito N, Qiao H, Yanagi T, Shinkuma S, Nishimura K, Suto A, et al. An annexin A1-FPR1 interaction contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. Sci Transl Med. 2014;6(245):245ra95.
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–50.
Nassif A, Bensussan A, Boumsell L, Deniaud A, Moslehi H, Wolkenstein P, et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. 2004;114(5):1209–15.
Olsson-Brown A, Yip V, Ogiji ED, Jolly C, Ressel L, Sharma A, et al. TNF-alpha-mediated keratinocyte expression and release of matrix metalloproteinase 9: putative mechanism of pathogenesis in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. J Invest Dermatol. 2023;143(6):1023–30 (e7).
Nassif A, Bensussan A, Dorothee G, Mami-Chouaib F, Bachot N, Bagot M, et al. Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol. 2002;118(4):728–33.
de Araujo E, Dessirier V, Lapree G, Valeyrie-Allanore L, Ortonne N, Stathopoulos EN, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20(2):107–12.
Villani AP, Rozieres A, Bensaid B, Eriksson KK, Mosnier A, Albert F, et al. Massive clonal expansion of polycytotoxic skin and blood CD8(+) T cells in patients with toxic epidermal necrolysis. Sci Adv. 2021. https://doi.org/10.1126/sciadv.abe0013.
Kinoshita M, Ogawa Y, Hama N, Ujiie I, Hasegawa A, Nakajima S, et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.aax2398.
Ueta M. Cold medicine-related Stevens-Johnson syndrome/toxic epidermal necrolysis with severe ocular complications-phenotypes and genetic predispositions. Taiwan J Ophthalmol. 2016;6(3):108–18.
Ueta M, Nishigaki H, Sotozono C, Kinoshita S. Downregulation of interferon-gamma-induced protein 10 in the tears of patients with Stevens-Johnson syndrome with severe ocular complications in the chronic stage. BMJ Open Ophthalmol. 2017;1(1): e000073.
Tangamornsuksan W, Chaiyakunapruk N, Somkrua R, Lohitnavy M, Tassaneeyakul W. Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(9):1025–32.
Administration USFaD. Table of Pharmacogenetic Associations. 2022 10/26/2022 [cited 2024 May 10, 2024]; Available from: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations
Mounzer K, Hsu R, Fusco JS, Brunet L, Henegar CE, Vannappagari V, et al. HLA-B*57:01 screening and hypersensitivity reaction to abacavir between 1999 and 2016 in the OPERA((R)) observational database: a cohort study. AIDS Res Ther. 2019;16(1):1.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–79.
Lai-Goldman M, Faruki H. Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genet Med. 2008;10(12):874–8.
Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–33.
Wu X, Liu W, Zhou W. Association of CYP2C9*3 with phenytoin-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. J Clin Pharm Ther. 2018;43(3):408–13.
Fohner AE, Rettie AE, Thai KK, Ranatunga DK, Lawson BL, Liu VX, et al. Associations of CYP2C9 and CYP2C19 pharmacogenetic variation with phenytoin-induced cutaneous adverse drug reactions. Clin Transl Sci. 2020;13(5):1004–9.
Nicoletti P, Bansal M, Lefebvre C, Guarnieri P, Shen Y, Pe’er I, et al. ABC transporters and the proteasome complex are implicated in susceptibility to Stevens-Johnson syndrome and toxic epidermal necrolysis across multiple drugs. PLoS One. 2015;10(6): e0131038.
Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015;16(6):475–93.
Roujeau J-C. Stevens-Johnson syndrome/toxic epidermal necrolysis (epithelial necrolysis). In: Shear NH, Dodiuk-Gad RP, editors. Advances in diagnosis and management of cutaneous adverse drug reactions: current and future trends. Singapore: Springer; 2019. p. 77–85.
Dodiuk-Gad RP, Chung WH, Shear NH. Adverse medication reactions. Clin Basic Immunodermatol. 2017;25:439–67.
Oakley AM, Krishnamurthy K. Stevens-Johnson Syndrome. StatPearls. Treasure Island; 2023.
Chen YL, Tsai TY, Pan LY, Tsai YJ, Chen SY, Hsiao CH, et al. Ocular manifestations and outcomes in children with Stevens-Johnson syndrome and toxic epidermal necrolysis: a comparison with adult patients. Am J Ophthalmol. 2023;256:108–17.
Misra R, Todd G, Esterhuizen J, Muloiwa R, Peter J, Lehloenya RJ. Stevens-Johnson syndrome and toxic epidermal necrolysis-associated dysgeusia: natural history and association with dysosmia. J Allergy Clin Immunol Pract. 2024;12(2):522–5.
Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y, Nakamura K, Kambara T, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients–treatment and outcome. Allergol Int. 2016;65(1):74–81.
Malik MN, Mujeeb Ullah A, Ahmad ME, Riaz R, Sadiq Syed TI. Pancytopenia in a patient with Stevens-Johnson syndrome: a case report with literature review. Cureus. 2019;11(5): e4702.
Weinkle A, Pettit C, Jani A, Keller J, Lu Y, Malachowski S, et al. Distinguishing Stevens-Johnson syndrome/toxic epidermal necrolysis from clinical mimickers during inpatient dermatologic consultation—a retrospective chart review. J Am Acad Dermatol. 2019;81(3):749–57.
Seminario-Vidal L, Kroshinsky D, Malachowski SJ, Sun J, Markova A, Beachkofsky TM, et al. Society of Dermatology Hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. 2020;82(6):1553–67.
Glasson N, De Sandre C, Pantet O, Reinhard A, Lambercy K, Sandu K, et al. Oropharyngolaryngeal manifestations in severe toxic epidermal necrolysis: a single-center’s retrospective case series. Int J Dermatol. 2023;62(11):1384–90.
Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63(2):117–24.
Maity S, Banerjee I, Sinha R, Jha H, Ghosh P, Mustafi S. Nikolsky’s sign: a pathognomic boon. J Family Med Prim Care. 2020;9(2):526–30.
Paquet P, Pierard GE, Quatresooz P. Novel treatments for drug-induced toxic epidermal necrolysis (Lyell’s syndrome). Int Arch Allergy Immunol. 2005;136(3):205–16.
Parisi R, Shah H, Shear NH, Ziv M, Markova A, Dodiuk-Gad RP. A review of bullous dermatologic adverse events associated with anti-cancer therapy. Biomedicines. 2023;11(2):323.
Wetter DA, Camilleri MJ. Clinical, etiologic, and histopathologic features of Stevens-Johnson syndrome during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2010;85(2):131–8.
Quinn AM, Brown K, Bonish BK, Curry J, Gordon KB, Sinacore J, et al. Uncovering histologic criteria with prognostic significance in toxic epidermal necrolysis. Arch Dermatol. 2005;141(6):683–7.
Tonellotto L, Seremet T, Vernez M, Guenova E, Kuonen F. Fast, bedside diagnosis of toxic epidermal necrolysis using ex vivo confocal laser scanning microscopy: a retrospective study. J Eur Acad Dermatol Venereol. 2024;38(1):182–5.
Grunwald P, Mockenhaupt M, Panzer R, Emmert S. Erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis—diagnosis and treatment. J Dtsch Dermatol Ges. 2020;18(6):547–53.
Wolf R, Lipozencic J. Shape and configuration of skin lesions: targetoid lesions. Clin Dermatol. 2011;29(5):504–8.
Iwai S, Sueki H, Watanabe H, Sasaki Y, Suzuki T, Iijima M. Distinguishing between erythema multiforme major and Stevens-Johnson syndrome/toxic epidermal necrolysis immunopathologically. J Dermatol. 2012;39(9):781–6.
Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schroder W, Roujeau JC, et al. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138(8):1019–24.
Albrahim L, Alasmari AA, Aleissa M. Pemphigus vulgaris mimicking Steven-Johnson syndrome/toxic epidermal necrolysis: report of an unusual case. Dermatol Reports. 2023;15(3):9649.
Sanfilippo E, Habeshian K, Cotton CH, Kirkorian AY. Severe reactive infectious mucocutaneous eruption mimicking drug-induced epidermal necrolysis triggered by norovirus. Pediatr Dermatol. 2024;41(1):84–6.
Abe R, Yoshioka N, Murata J, Fujita Y, Shimizu H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann Intern Med. 2009;151(7):514–5.
Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3):985–96.
Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Invest Dermatol. 2017;137(5):1065–73.
Fujita Y, Yoshioka N, Abe R, Murata J, Hoshina D, Mae H, et al. Rapid immunochromatographic test for serum granulysin is useful for the prediction of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol. 2011;65(1):65–8.
Weinborn M, Barbaud A, Truchetet F, Beurey P, Germain L, Cribier B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int J Dermatol. 2016;55(11):1225–33.
Sadek M, Iqbal O, Siddiqui F, Till S, Mazariegos M, Campbell E, et al. The role of IL-13, IL-15 and granulysin in the pathogenesis of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Clin Appl Thromb Hemost. 2021;27:1076029620950831.
Nakajima S, Watanabe H, Tohyama M, Sugita K, Iijima M, Hashimoto K, et al. High-mobility group box 1 protein (HMGB1) as a novel diagnostic tool for toxic epidermal necrolysis and Stevens-Johnson syndrome. Arch Dermatol. 2011;147(9):1110–2.
Nwikue G, Olsson-Brown A, Aboheimed N, Yip V, Jolly C, Luchian A, et al. TNF-alpha induced extracellular release of keratinocyte high-mobility group box 1 in Stevens-Johnson syndrome/toxic epidermal necrolysis: Biomarker and putative mechanism of pathogenesis. J Dermatol. 2023;50(9):1129–39.
Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014;2(1):21–33.
Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88(1):60–8.
Goldman JL, Chung WH, Lee BR, Chen CB, Lu CW, Hoetzenecker W, et al. Adverse drug reaction causality assessment tools for drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: room for improvement. Eur J Clin Pharmacol. 2019;75(8):1135–41.
Gronich N, Maman D, Stein N, Saliba W. Culprit medications and risk factors associated with Stevens-Johnson syndrome and toxic epidermal necrolysis: population-based nested case-control study. Am J Clin Dermatol. 2022;23(2):257–66.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS One. 2011;6(12): e28096.
Copaescu A, Gibson A, Li Y, Trubiano JA, Phillips EJ. An updated review of the diagnostic methods in delayed drug hypersensitivity. Front Pharmacol. 2020;11: 573573.
Copaescu AM, Trubiano JA. In vitro/ex vivo assays for severe cutaneous drug reactions. J Allergy Clin Immunol. 2023;152(1):39–41.
Novack DE, Braskett M, Worswick SD, Adler BL. Drug patch testing in Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review. Ann Allergy Asthma Immunol. 2023;130(5):628–36.
Whitley L, Honsinger R. Drug patch testing for severe cutaneous adverse reactions: Not in the United States? Ann Allergy Asthma Immunol. 2023;130(5):538–9.
Vieira R, Goncalo M, Figueiredo A. FS09.5 Patch testing with allopurinol and oxypurinol in drug eruptions. Contact Dermatitis. 2004;50:156.
Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168(3):555–62.
Thaiwat S, Rojanapanthu P. Cutaneous adverse drug eruption: the role of drug patch testing. Int J Dermatol. 2023;62(1):108–14.
Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome—alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43(9):1027–37.
Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59(8):809–20.
Kumkamthornkul P, Udnaen S, Tansit T, Tuchinda P, Srinoulprasert Y. Evaluation of a lymphocyte transformation test and cytokine detection assay to identify phenytoin and carbamazepine provoked DRESS or SJS/TEN in epilepsy patients. Int Immunopharmacol. 2018;63:204–10.
Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. Causes and treatment outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in 82 adult patients. Korean J Intern Med. 2012;27(2):203–10.
Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197–204.
Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Use of SCORTEN to accurately predict mortality in patients with toxic epidermal necrolysis in the United States. Arch Dermatol. 2004;140(7):890–2.
Dobry AS, Himed S, Waters M, Kaffenberger BH. Scoring assessments in Stevens-Johnson syndrome and toxic epidermal necrolysis. Front Med (Lausanne). 2022;9: 883121.
Koh HK, Fook-Chong SMC, Lee HY. Improvement of mortality prognostication in patients with epidermal necrolysis: the role of novel inflammatory markers and proposed revision of SCORTEN (Re-SCORTEN). JAMA Dermatol. 2022;158(2):160–6.
Noe MH, Rosenbach M, Hubbard RA, Mostaghimi A, Cardones AR, Chen JK, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis-ABCD-10. JAMA Dermatol. 2019;155(4):448–54.
Duplisea MJ, Roberson ML, Chrisco L, Strassle PD, Williams FN, Ziemer CM. Performance of ABCD-10 and SCORTEN mortality prediction models in a cohort of patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. J Am Acad Dermatol. 2021;85(4):873–7.
Hama N, Sunaga Y, Ochiai H, Kokaze A, Watanabe H, Kurosawa M, et al. Development and validation of a novel score to predict mortality in Stevens-Johnson syndrome and toxic epidermal necrolysis: CRISTEN. J Allergy Clin Immunol Pract. 2023;11(10):3161–8 (e2).
Waters M, Dobry A, Le ST, Shinkai K, Beachkofsky TM, Davis MDP, et al. Development of a skin-directed scoring system for Stevens-Johnson syndrome and epidermal necrolysis: a delphi consensus exercise. JAMA Dermatol. 2023;159(7):772–7.
Caux F, Chosidow O, Philippon C, Roujeau JC, Revuz J. Increased serum and blister fluid levels of creatine kinase in patients with toxic epidermal necrolysis. Br J Dermatol. 1994;130(3):337–41.
Yun SJ, Choi MS, Piao MS, Lee JB, Kim SJ, Won YH, et al. Serum lactate dehydrogenase is a novel marker for the evaluation of disease severity in the early stage of toxic epidermal necrolysis. Dermatology. 2008;217(3):254–9.
Watanabe Y, Watanabe T, Yamaguchi Y. Anti-SS-A antibody is a potential predictor of severe Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective cohort study. J Am Acad Dermatol. 2024;90(2):385–7.
Shanbhag SS, Chodosh J, Fathy C, Goverman J, Mitchell C, Saeed HN. Multidisciplinary care in Stevens-Johnson syndrome. Ther Adv Chronic Dis. 2020;11:2040622319894469.
Beck A, Cooney R, Gamelli RL, Mosier MJ. Predicting mechanical ventilation and mortality: early and late indicators in Steven-Johnson syndrome and toxic epidermal necrolysis. J Burn Care Res. 2016;37(1):e47–55.
Hung CC, Liu WC, Kuo MC, Lee CH, Hwang SJ, Chen HC. Acute renal failure and its risk factors in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Nephrol. 2009;29(6):633–8.
Krumlovsky FA, Del Greco F, Herdson PB, Lazar P. Renal disease associated with toxic epidermal necrolysis (Lyell’s disease). Am J Med. 1974;57(5):817–25.
Sunaga Y, Hama N, Ochiai H, Kokaze A, Lee ES, Watanabe H, et al. Risk factors for sepsis and effects of pretreatment with systemic steroid therapy for underlying condition in SJS/TEN patients: Results of a nationwide cross-sectional survey in 489 Japanese patients. J Dermatol Sci. 2022;107(2):75–81.
Enescu CD, Elder AJ, Deirawan H, Moossavi M. To debride or not to debride: a review of wound management for Stevens-Johnson syndrome and toxic epidermal necrolysis. Cureus. 2024;16(3): e55350.
Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71(5):941–7.
Ng QX, De Deyn M, Venkatanarayanan N, Ho CYX, Yeo WS. A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. 2018;11:135–42.
Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maitre B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163(4):847–53.
Hsieh MH, Watanabe T, Aihara M. Recent dermatological treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan. Front Med (Lausanne). 2021;8: 636924.
Pasricha JS. Corticosteroids in toxic epidermal necrolysis. Indian J Dermatol Venereol Leprol. 2008;74(5):493 (author reply -5).
Kevin R, Munnerlyn B, Kahn S. 54 prophylactic antibiotics are unnecessary for routine CO2 laser burn scar treatment. J Burn Care Res. 2023;44(Supplement_2):S24.
Tsai TY, Huang IH, Chao YC, Li H, Hsieh TS, Wang HH, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;84(2):390–7.
Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33–40.
Bachot N, Revuz J, Roujeau JC. Intravenous immunoglobulin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis: a prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol. 2003;139(1):33–6.
Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil. 2004;25(3):246–55.
Zhang S, Tang S, Li S, Pan Y, Ding Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a systemic review. J Dermatolog Treat. 2020;31(1):66–73.
Jeong MS, Choi YY, Ahn YH, Lee K, Park JS, Suh DI. Etanercept treatment for pediatric toxic epidermal necrolysis induced by deflazacort: a case report and literature review. Front Immunol. 2024;15:1342898.
Peng YT, Xiong JX, Wei B, Li H, Xu J, Chen AJ, et al. Tumor necrosis factor-alpha inhibitor for successful treatment of toxic epidermal necrolysis with severe infection: a case series. J Int Med Res. 2024;52(1):3000605231223059.
Miller MM, Kamath S, Hughes M, Harter N, Luu M. Evaluation of Etanercept for treatment of reactive infectious mucocutaneous eruption. JAMA Dermatol. 2021;157(2):230–2.
Mukherjee EM, Phillips EJ. Where to place etanercept and combination treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis? Ann Allergy Asthma Immunol. 2022;129(3):269–70.
Jacobsen A, Olabi B, Langley A, Beecker J, Mutter E, Shelley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. 2022;3(3): CD013130.
Zhang J, Lu CW, Chen CB, Wang CW, Chen WT, Cheng B, et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol Pract. 2022;10(5):1295–304 (e6).
Ingen-Housz-Oro S, Schmidt V, Ameri MM, Abe R, Brassard A, Mostaghimi A, et al. Post-acute phase and sequelae management of epidermal necrolysis: an international, multidisciplinary DELPHI-based consensus. Orphanet J Rare Dis. 2023;18(1):33.
Dodiuk-Gad RP, Olteanu C, Feinstein A, Hashimoto R, Alhusayen R, Whyte-Croasdaile S, et al. Major psychological complications and decreased health-related quality of life among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2016;175(2):422–4.
Hoffman M, Chansky PB, Bashyam AR, Boettler MA, Challa N, Dominguez A, et al. Long-term physical and psychological outcomes of Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2021;157(6):712–5.
O’Reilly P, Kennedy C, Meskell P, Coffey A, Delaunois I, Dore L, et al. The psychological impact of Stevens-Johnson syndrome and toxic epidermal necrolysis on patients’ lives: a Critically Appraised Topic. Br J Dermatol. 2020;183(3):452–61.
Lifetime burden of Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2023;189(5):e92. https://doi.org/10.1093/bjd/ljad352. PMID: 37879747.
Liu Y, Li Q, Chu C, Zhou Y. Insights into Stevens-Johnson syndrome/toxic epidermal necrolysis lifetime burden: assessing life expectancy, healthcare costs and quality of life. Br J Dermatol. 2023;189(5):648.
Buckey TM, Ferreira AL, Grant-Kels JM. Ethical considerations in the management of drug severe cutaneous adverse reactions. J Am Acad Dermatol. 2024. https://doi.org/10.1016/j.jaad.2024.01.011.
Roujeau JC. Stevens-Johnson Syndrome and toxic epidermal necrolysis: improving the support to victims. Drug Saf. 2013;36(2):145–6.
Phillips E, Shear N. Table 1 - Reported genetic associations with cutaneous adverse drug reactions and other immune-mediated reactions. In: Shear N, editor. Litt’s Drug Eruption & Reaction Manual: Taylor & Francis Group, LLC; 2024. p. 491-501.
Wu PC, Chen WT, Huang IH, Chen CB, Wang CW, Tai CC, et al. Human leukocyte antigens and sulfamethoxazole/cotrimoxazole-induced severe cutaneous adverse reactions: a systematic review and meta-analysis. JAMA Dermatol. 2024;160(5):525–34.
Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57(9).
Ross A, Shoff HW. Staphylococcal scalded skin syndrome. StatPearls. Treasure Island (FL); 2024.
Hafsi W, Badri T. Erythema Multiforme. StatPearls. Treasure Island (FL); 2024.
White KD, Abe R, Ardern-Jones M, Beachkofsky T, Bouchard C, Carleton B, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract. 2018;6(1):38–69.
Gandelman JS, Kim EY, Grzegorczyk AM, Zejnullahu K, Edson RS. Mycoplasma pneumoniae-induced rash and mucositis in a previously healthy man: a case report and brief review of the literature. Open Forum Infect Dis. 2020;7(10): ofaa437.
Stirton H, Shear NH, Dodiuk-Gad RP. Drug Reaction with Eosinophilia and Systemic Symptoms (DReSS)/Drug-Induced Hypersensitivity Syndrome (DiHS)-Readdressing the DReSS. Biomedicines. 2022;10(5):999.
Parisi R, Shah H, Navarini AA, Muehleisen B, Ziv M, Shear NH, et al. Acute generalized exanthematous pustulosis: clinical features, differential diagnosis, and management. Am J Clin Dermatol. 2023;24(4):557–75.
Abdelmouttalib A, Meziane M, Senouci K. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus: two cases report. Pan Afr Med J. 2021;38:236.
Sethy M, Padhan P, Abirami C, Maikap D. Rowell’s syndrome: a case report and literature overview. Indian Dermatol Online J. 2021;12(4):608–10.
Torchia D, Romanelli P, Kerdel FA. Erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis associated with lupus erythematosus. J Am Acad Dermatol. 2012;67(3):417–21.
Lee D, Waseh S, Motaparthi K, Hsu S. Rowell syndrome in a middle-aged woman: a case report. Cureus. 2023;15(5): e39631.
Novak-Bilic G, Vucic M, Japundzic I, Mestrovic-Stefekov J, Stanic-Duktaj S, Lugovic-Mihic L. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57(4):713–20.
Strong Rodrigues K, Oliveira-Ribeiro C, de Abreu Fiuza Gomes S, Knobler R. Cutaneous graft-versus-host disease: diagnosis and treatment. Am J Clin Dermatol. 2018;19(1):33–50.
Auyeung J, Lee M. Successful treatment of Stevens-Johnson syndrome with cyclosporine and corticosteroid. Can J Hosp Pharm. 2018;71(4):272–5.
Kim KJ, Lee DP, Suh HS, Lee MW, Choi JH, Moon KC, et al. Toxic epidermal necrolysis: analysis of clinical course and SCORTEN-based comparison of mortality rate and treatment modalities in Korean patients. Acta Derm Venereol. 2005;85(6):497–502.
Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007;87(2):144–8.
Barrera-Ochoa CA, Marioni-Manriquez S, Cortazar-Azuaje AM, Quijada-Ucelo ZM, Saba-Mussali AJ, Vega-Memije ME. Use of intravenous immunoglobulins and systemic corticosteroids in patients with toxic epidermal necrolysis: experience of a hospital in Mexico City. Actas Dermosifiliogr. 2022;113(3):294–9.
Struzyna J, Surowiecka A, Korzeniowski T, Piszczek J, Korulczyk P, Drozd L, et al. Immunomodulatory treatment of Lyell’s syndrome: a simultaneous plasmapheresis and intravenous immunoglobulins therapy. J Burn Care Res. 2022;43(6):1394–8.
Lee HY, Walsh SA, Creamer D. Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol. 2017;177(4):924–35.
Olteanu C, Shear NH, Chew HF, Hashimoto R, Alhusayen R, Whyte-Croasdaile S, et al. Severe physical complications among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Drug Saf. 2018;41(3):277–84.
Saeed H, Mantagos IS, Chodosh J. Complications of Stevens-Johnson syndrome beyond the eye and skin. Burns. 2016;42(1):20–7.
Ueta M, Inoue C, Nakata M, Sotozono C, Kim MK, Wakamatsu T, et al. Severe ocular complications of SJS/TEN and associations among pre-onset, acute, and chronic factors: a report from the international ophthalmology collaborative group. Front Med (Lausanne). 2023;10:1189140.
Acknowledgements
E.M. is funded by a Dermatologist Investigator Research Fellowship (DIRF) from the Dermatology Foundation and a National Institutes of Health—National Institute for General Medical Sciences (NIH NIGMS) training grant (5T32GM007569). E.J.P. receives funding from the National Institutes of Health (R01HG010863, R01AI152183, U01AI154659, R13AR074889-01) and National Health and Medical Research Council of Australia (NHMRC) not directly related to the submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
R.P.D.G. consults for Janssen, Sanofi, AbbVie, Novartis, Pfizer, La Roche-Posay, Dexcel, Eli Lilly, and Devintec Pharma. E.J.P. receives royalties and consulting fees from UpToDate and consulting fees from Janssen, Verve, Esperion, Servier, and RAPT independent from the submitted work. R.P., H.S., E.M. have no relevant conflicts of interest to disclose.
Funding
No specific funding was received for the preparation of this manuscript.
Ethics Approval
Not applicable.
Patient Consent to Participate/Publish
Patient consent was received for photo publication.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the study conceptualization, literature search and data analysis, writing—original draft preparation, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data derived from public resources and made available with the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shah, H., Parisi, R., Mukherjee, E. et al. Update on Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Diagnosis and Management. Am J Clin Dermatol (2024). https://doi.org/10.1007/s40257-024-00889-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40257-024-00889-6