Abstract
Objectives
Adverse reactions to the COVID-19 vaccines have been of interest since their emergency authorization. Cutaneous manifestations of the vaccines are not well studied. We aimed to characterize cutaneous reactions to the Moderna (mRNA-1273) and the Pfizer-BioNTech (BNT162b2) COVID-19 vaccines on a large, national scale.
Methods
The Vaccine Adverse Event Reporting System was filtered for cutaneous and hair and nail reactions to the COVID-19 vaccines. Patient demographics and past medical histories, vaccine manufacturer and dosing, symptom timing, reaction location, and patient outcomes were extracted from each report.
Results
As of December 24, 2021, there were 67,273 cutaneous reactions to all COVID-19 vaccines, with most patients receiving the Moderna (mRNA-1273) or Pfizer-BioNTech (BNT162b2) vaccines. The most common reactions overall were injection-site reaction, urticaria, and papular rash, with injection-site reaction more common after the Moderna (mRNA-1273) vaccine, and all other cutaneous reactions more common after the Pfizer-BioNTech (BNT162b2) vaccine. Patients with past histories of psoriasis, urticaria, and local site reactions to a vaccine were more likely to report these same symptoms after the COVID-19 vaccine.
Conclusion
Patients should be counseled about these potential dermatologic reactions to the COVID-19 vaccines. Most occur within the first few days after vaccination, and are mild and self-limiting. Patients should therefore be encouraged that it is safe to receive the COVID-19 vaccine from a dermatological perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cutaneous reactions to the COVID-19 vaccinations are mild, and most occur within the first few days after vaccination. |
Injection site reaction is most common overall, and less frequent with Pfizer vs. Moderna vaccines. |
Patients with past history of psoriasis or urticaria are more likely to experience flares after vaccination compared to the general population, and the Moderna vaccine may lessen this risk. |
1 Introduction
The Food and Drug Administration (FDA) issued Emergency Use Authorizations for the Moderna (mRNA-1273) and Pfizer-BioNTech (BNT162b2) COVID-19 vaccines in 12/2020 [1], and for the Janssen (JNJ-78436735) vaccine in 2/2021 [2], which has been essential to limit disease transmission and severity. To date, clinical trials have demonstrated fatigue, headache, and myalgia, and local injection-site reactions, after the first and second doses of the Moderna and Pfizer vaccines [3, 4], while registry-based studies reported delayed large local reactions, urticarial, and morbilliform eruptions to both vaccines [1, 5]. However, these studies were relatively small, making generalizability, and understanding cutaneous reactions to the COVID-19 vaccines on a large scale, limited.
The FDA, together with the Centers for Disease Control and Prevention, co-administer the Vaccine Adverse Event Reporting System (VAERS) database, allowing for continual safety monitoring of approved vaccinations. Healthcare professionals, patients, caregivers, and vaccine manufacturers can report unanticipated or unusual events after vaccination [6], allowing for the collection of vaccine safety data on a large and national scale. We aimed to analyze the VAERS database to examine morphology, timing, and differences in cutaneous reactions to the Moderna and Pfizer COVID-19 vaccines, to ease patient hesitancy and guide vaccine counseling.
2 Methods
The 2020–2021 VAERS public data set was downloaded from https://vaers.hhs.gov/index.html on 2 January 2022, containing all adverse event reports processed as of 24 December 2021. Since all data was publicly available and deidentified, patient informed consent and institutional review board approval were not required. Data was filtered for reported adverse events to the COVID-19 vaccine, including the Moderna vaccine, the Pfizer-BioNTech vaccine, the Janssen vaccine, and vaccines of unspecified manufacturer. Reports were filtered for cutaneous and hair and nail reactions using automated query of standardized, preferred terms from the VAERS Medical Dictionary for Regulatory Activities (Tables 2, 3). Free-text descriptions were coded for location, systemic symptoms, past medical history, past dermatologic history, and adverse events to prior vaccinations.
For each VAERS report, age, sex, past medical, dermatologic, and vaccine reaction histories, vaccine manufacturer and dose, days to symptom onset, reaction location, and patient outcomes were collected. A descriptive analysis for all vaccines and doses was conducted to characterize the reported adverse events. A comparative (Pfizer-BioNTech vaccine vs Moderna vaccine) analysis was conducted, overall, and for the first, second, and third doses. Univariable and multivariable analyses were performed using Microsoft Excel (Microsoft, Seattle, WA, USA) and SAS Software (SAS Studio Release 3.8, Cary, NC, USA) with a two-sided α-value of 0.05 to assess significance.
3 Results
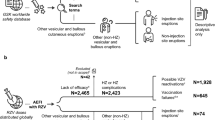
There were 62,386 patients with one or more cutaneous reactions (67,273 total) to a COVID-19 vaccination in the VAERS database. Average patient age was 48.42 years (standard deviation [SD]: 17.60) with female predominance (86.05%). Psoriasis (0.85%) and urticaria (0.50%) were the most common past dermatologic conditions (Table 1). The majority of patients received the Moderna vaccine (63.64%) and the Pfizer-BioNTech vaccine (26.23%). Overall, the most common cutaneous reactions were injection-site reaction (59.73%), urticaria (32.11%), papular rash (4.90%), angioedema (1.26%), petechiae (1.19%), and vesicular rash (1.13%), occurring at a median 2.0 (interquartile range [IQR] 6.0), 1.0 (IQR 6.0), 4.0 (IQR 7.0), 1.0 (IQR 4.0), 4.0 (IQR 12.0), and 3.0 (IQR 9.0) days after vaccination, respectively. The most common systemic symptoms were fever (14.39%), headache (14.30%), and fatigue (13.01%), with only 2.88% of patients reporting myalgia. The proportion of patients with versus without systemic symptoms was greater in those reporting injection-site reactions (37.96% vs 28.69%; p < 0.0001), chilblains (36.92% vs 34.18%; p = 0.6415), petechiae (35.8% vs 34.2%; p = 0.3287), urticaria (37.65% vs 34.17%; p = 0.1773), and erythromelalgia (45.0% vs 34.18%; p = 0.3078). Few patients were hospitalized (1.45%), disabled (0.61%), or died (0.04%) (Table 2).
There were 45,316 cutaneous reactions reported with the Moderna vaccine. The most common overall were injection-site reaction (70.40%), urticaria (23.74%), and papular rash (4.57%) (Table 2), and after the first (72.5%, 22.35%, 4.73%, respectively), second (65.54%, 25.92%, 4.53%, respectively), and third (50.08%, 44.60%, 3.48%, respectively) doses. Median time to cutaneous reaction was faster for the second and third (both 1.0 days) versus first (7.0 days) vaccine doses (Table 3).
There were 18,622 reports of cutaneous reactions after the Pfizer vaccine. The most common overall were urticaria (51.39%), injection-site reaction (33.84%), and papular rash (5.72%) (Table 2), which was consistent after the first (56.42%, 28.79%, 6.15%, respectively), and second (47.88%, 36.58%, 5.49%, respectively) doses; however, after the third dose, injection-site reaction (60.34%) was more frequent than urticaria (30.09%). Median time to cutaneous reaction was the same (1.0 days) for all three doses (Table 3).
Injection-site reactions were more common with the Moderna vaccine than the Pfizer vaccine (70.40% vs 33.84%, respectively), while urticaria (23.74% vs 51.39%), papular rash (4.57% vs 5.72%), angioedema (0.87% vs 2.21%), petechiae (0.68% vs 2.07%), vesicular rash (0.625% vs 1.97%), and psoriasis (0.41% vs 1.32%) were less common with the Moderna vaccine compared with the Pfizer vaccine, respectively (all p < 0.0001) (Table 2). The rate of injection-site reactions was highest after the first Moderna vaccine dose, and rates of urticaria and papular rash were both highest after the first Pfizer vaccine dose, compared with all other Moderna vaccine and Pfizer vaccine doses (all p < 0.0001) (Table 3). Overall, mean time to cutaneous reaction was slower for the Moderna vaccine (6.13 days, SD 15.46) compared with the Pfizer vaccine (4.77 days, SD 16.68) (p < 0.0001). Overall, most patients reported cutaneous reactions within the first two days of vaccination decreasing thereafter, except for a smaller peak approximately 7–10 days after the first and third Moderna vaccine doses (Fig. 1). For systemic reactions, arm tingling/numbness (0.97% vs 1.64%; p < 0.0001) and nausea (6.10% vs 6.84%; p = 0.0005) were less common with the Moderna vaccine than the Pfizer vaccine, respectively, while fever (13.96% vs 13.34%; p = 0.0412) and chills (10.12% vs 9.60%; p = 0.0467) were more common with the Moderna vaccine than the Pfizer vaccine, respectively (Table 2).
Patients with versus without history of psoriasis were more likely to report psoriatic rashes with COVID-19 vaccination (26.83% vs 0.48%) (odds ratio [OR] 75.36; 95% confidence interval [CI] 60.87–93.29; p < 0.0001). Patients with versus without past dermatologic history of urticaria were more likely to report urticarial eruptions (69.71% vs 31.92%) (OR 4.91; 95% CI 3.89–6.19; p < 0.0001), and those with versus without history of urticarial reaction to a prior vaccine were more likely to report urticarial eruptions (67.96% vs 32.00%) (OR 4.51; 95% CI 3.36–6.04; p < 0.0001). Patients with versus without history of prior local site reaction to a vaccine were more likely to report injection-site reactions (3.08% vs 1.30%) (OR 2.40; 95% CI 2.14–2.70; p < 0.0001).
4 Discussion
This is the largest COVID-19 vaccination cutaneous study to date. We found that the most common cutaneous adverse reaction to the COVID-19 vaccination was injection-site reaction. Compared with the Moderna vaccine, patients who received the Pfizer vaccine were more likely to experience all other cutaneous reactions, except injection-site reactions, which were more frequently seen after the Moderna vaccine. Overall, most cutaneous reactions occurred shortly after vaccination, with very few reports 10 days or greater after vaccination.
Our findings were similar to a registry-based study of 414 cases of cutaneous reactions to the Moderna COVID-19 vaccine and the Pfizer COVID-19 vaccine [1], in which delayed large local reaction (60.1%), local injection-site reaction (54.2%), and urticaria (6.7%) were most common after the Moderna vaccine, while local injection-site reaction (22.5%) and urticaria (19.7%) were most common after the Pfizer vaccine. Morbilliform rash and erythromelalgia occurred in 5.2% and 3.2% of patients who were vaccinated with the Moderna vaccine, respectively, and 12.7% and 4.2% with the Pfizer vaccine, respectively, while we observed lower rates, specifically 0.20% and 0.02% of the Moderna vaccine patients, respectively, and 0.50% and 0.07% of the Pfizer vaccine patients, respectively (Table 4). Median time from first vaccination to onset of cutaneous symptoms was 7 days in that study, in two clusters, days 1–3 and then days 7–8, similar to the timing in our study. Furthermore, in a nationwide, multicenter, cross-sectional, observational study of 405 cutaneous reactions to the Moderna vaccine, the Pfizer vaccine, or the AstraZeneca vaccine [5], the most common reactions were injection-site reaction (32.1%), urticaria (14.6%), morbilliform rash (8.9%), papulovesicular rash (6.4%), pityriasis rosea-like reaction (4.9%), and purpuric reaction (4%), occurring on average 5.1 (SD 4.4) days after vaccination for average duration of 12.2 (SD 13.1) days. Our findings taken together with results from previous studies suggest that cutaneous reactions to the COVID-19 vaccines are generally mild, with the majority being delayed or local injection-site reactions, which are not specific to the COVID-19 vaccine, but also commonly encountered with other routinely administered vaccine types, such as the influenza vaccine [7]. Furthermore, the death rate in our study (0.04%) was lower than death rate (0.35%) reported in an analysis of the VAERS database for reports of adverse events after the recombinant hemagglutinin quadrivalent influenza vaccine [8]. Therefore, patients can be counseled that COVID-19 reactions are mild and have lower risk of serious adverse events compared with other commonly administered vaccines.
We found that while < 1% of patients reported psoriatic reactions in association with COVID-19 vaccination, occurring 5.0 days after vaccination, patients with past histories of psoriasis were 56 times more likely to report psoriatic rashes. Psoriatic flares in response to the COVID-19 vaccine were reported in case reports [9,10,11,12,13,14], and in some larger studies [1, 15]. For example, in a study of patients with known history of psoriasis (51 vaccinated and 32 not vaccinated against COVID-19) [15], vaccinated versus unvaccinated patients were almost eight times more likely to experience psoriasis flares (p = 0.047), at mean 9.3 ± 4.3 days following vaccination. In a registry-based study of 414 cases of cutaneous reactions to COVID-19 vaccines [1], 22.2% (2/9) of patients with history of psoriasis experienced a flare after vaccination. It is theorized that mRNA COVID-19 vaccines may activate inflammatory pathways, leading to elevated levels of interleukin-6 and Th-17 cells, resulting in new onset psoriasis or flares [15]. In a survey of 50 vaccinated psoriatic patients treated with biologics [16], only one patient reported a psoriasis exacerbation after the vaccine, suggesting that that vaccine-induced flares may be limited in patients receiving immunosuppressants. Dermatologists should counsel patients with psoriasis, especially those that are untreated, that flares may occur with COVID-19 vaccination.
We found that urticaria was the second most common cutaneous reaction overall, and patients with a history of urticaria or a previous urticarial reaction to a vaccine were both two times more likely to report urticarial eruptions in response to the vaccine. Urticarial reactions to both the Moderna vaccine and the Pfizer vaccine have been reported. In one study [1], none of the urticarial reactions were classified as an immediate hypersensitivity rash. In another study [17], the urticarial reactions were delayed (>4 hours after vaccination) and widespread. In a study of 405 patients with cutaneous reactions to the Moderna vaccine, the Pfizer vaccine, or the AstraZeneca vaccine, urticaria (14.6%) was the second most common reaction after injection-site reactions (32.1%) [5]. A history of urticaria was present in 18.6% of patients with acute urticarial reactions to the COVID-19 vaccine. In a retrospective analysis of 714 patients who received the Pfizer COVID-19 vaccine, positive autologous serum skin test result (OR 5.54, 95% CI 2.36–13.02; p < 0.001), overall allergic comorbidities (OR 6.13, 95% CI 2.52–14.89; p = 0.001) and basopenia (OR 2.81, 95% CI 1.17–6.72; p = 0.020) were positively associated with probability of chronic spontaneous urticaria (CSU) relapse within 3 months after vaccination, while there were no associations between these variables and new-onset CSU [18]. Larger studies are needed to confirm the association between COVID-19 vaccination and urticarial remission, and to identify the driving factors behind this interaction.
Patients with a previous history of local site reaction to a vaccine were more than two times more likely to report injection-site reactions to the COVID-19 vaccine. While patients may be hesitant to receive the vaccine in fear of developing ‘COVID arm,’ or delayed, large, injection-site reactions that begin approximately 1 week post-vaccination and persist for several days [19], our findings suggest that patients who routinely received previous vaccinations without such reactions are less likely to experience ‘COVID arm,’ and therefore should be counseled accordingly.
We found that the most common systemic reactions in patients reporting cutaneous reactions were fever, headache, and fatigue. Patients with injection-site reactions, chilblains, petechiae, urticaria, and erythromelalgia developed systemic symptoms at higher proportions compared with all other patients. Our data aligns with results from phase III clinical trials, in which fatigue, headache, and myalgia were the most commonly reported systemic reactions to the Moderna vaccine and the Pfizer vaccine [3, 4]; however, very few patients reported myalgia in our study. We also found high rates of chills (10.19%) and nausea (6.41%), which is similar to findings in the registry-based study of 414 cutaneous reactions to mRNA COVID-19 vaccines (15.8% and 11.3%, respectively) [1]. However, they also reported higher rates of arthralgia (13.8%) than observed in our study (2.20%). We observed several less commonly reported reactions, including chilblains (0.09%), erythromelalgia (0.03%), and pityriasis-rosea (0.37%). Interestingly, these reactions have previously been observed with the COVID-19 vaccines [1, 20, 21], and to the SARS-CoV-2 infection itself [22,23,24].
This study is subject to several limitations. Data inputted into the VAERS database is voluntary and can be self-reported, which is subject to reporting biases. Failure to recognize unusual or unexpected reactions following vaccination, especially by non-healthcare providers, may result in underreporting of adverse events, while providers who input reports may be more likely to submit severe or unusual cases, making it challenging to determine true incidence or prevalence [6, 25]. Definitive diagnoses can be lacking in reports submitted by non-medical personnel. There is no category in the VAERS report form prompting the reporter to disclose whether they are a healthcare professional, patient, caregiver, or vaccine manufacturer; therefore, information regarding the number of events submitted by reporter title cannot be deduced. We observed a female predominance (86.05%) of cutaneous reactions to the COVID-19 vaccines, which was similar to other studies, reporting 90% [1] and 80.2% [5] females. Our results must therefore be interpreted with caution in male patients, and future studies should discern whether there is a true sex-related difference in cutaneous reactions to the vaccine, or whether there is a sex bias in reporting. Analysis is restricted to information included in reports, which may be incomplete [26], and causal associations cannot be made. All data is from United States reports, preventing analysis of reactions to COVID-19 vaccination on a global level. Given the volume of reports, data contained in free-text descriptions, including skin area, systemic symptoms, past medical and dermatologic history, and adverse events to prior vaccinations, were coded, and it is possible that some cases may have been missed. Participants may have more than one adverse event reported per VAERS identification number, and therefore, information such as hospitalization, mortality rates, and disability, may not be reflective of dermatologic manifestations of the vaccine, and could be skewed by co-reporting of other, more serious adverse events. Head-to-head comparisons with the Janssen vaccine were omitted due to limited number of cases.
5 Conclusion
Our study shows that most cutaneous reactions to the COVID-19 vaccines are injection-site reactions, which are generally mild, self-limiting, and non-life threatening. Patients with previous histories of vaccine local site reaction are more likely to have an injection-site reaction. Administration of the Pfizer vaccine may decrease this risk. Patients with psoriasis should be warned that flares may occur with COVID-19 vaccination, and patients with past urticarial reactions should be counseled about risk of urticarial eruptions with vaccination. Administration of the Moderna vaccine to these groups may lessen likelihood of flares. Since cutaneous reactions >10 days after vaccination are rare, reactions more than 10 days post-vaccination warrant a comprehensive history and full skin examination to look for other etiologies. Dermatologists should be aware of these reactions to the COVID-19 vaccine, and should counsel their patients on the safety as well as benefits of receiving the vaccine, which may help curb vaccine hesitancy. Future studies should analyze cutaneous reactions on a global scale, examining diverse ethnic and racial populations, as well as men and children.
References
McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55.
Shay DK. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine—United States, March–April 2021. MMWR Morbidity and mortality weekly report. 2021;70.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New England Journal of Medicine. 2020.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020.
Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-COV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–52.
Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33(36):4398–405.
Moro PL, Woo EJ, Marquez P, et al. Monitoring the safety of high-dose, trivalent inactivated influenza vaccine in the vaccine adverse event reporting system (VAERS), 2011–2019. Vaccine. 2020;38(37):5923–6.
Woo EJ, Moro PL. Postmarketing safety surveillance of quadrivalent recombinant influenza vaccine: reports to the vaccine adverse event reporting system. Vaccine. 2021;39(13):1812–7.
Krajewski P, Matusiak J. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35(10): e632.
Piccolo V, Russo T, Mazzatenta C, et al. COVID vaccine‐induced pustular psoriasis in patients with previous plaque type psoriasis. J Eur Acad Dermatol Venereol. 2022.
Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021;17:1–3.
Durmaz I, Turkmen D, Altunisik N, et al. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID‐19 vaccine: A report of three cases. Dermatologic Therapy.e15331.
Bostan E, Elmas L, Yel B, et al. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID-19 vaccines: a report of two cases. Dermatol Ther. 2021. https://doi.org/10.1111/dth.15110.
Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021. https://doi.org/10.1111/jdv.17582.
Huang Y-W, Tsai T-F. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 2021;8:2768.
Musumeci ML, Caruso G, Trecarichi AC, et al. Safety of SARS-CoV-2 vaccines in psoriatic patients treated with biologics: a real life experience. Dermatol Ther. 2022;35(1): e15177.
Pitlick MM, Joshi AY, Gonzalez-Estrada A, et al., editors. Delayed systemic urticarial reactions following mRNA COVID-19 vaccination. Allergy and Asthma Proceedings; 2022: OceanSide Publications, Inc.
Magen E, Yakov A, Green I, et al., editors. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer-BioNTech) vaccination against SARS-CoV-2. Allergy & Asthma Proceedings; 2022.
Ramos CL, Kelso JM. “COVID Arm”: very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021;9(6):2480–1.
Marcantonio‐Santa Cruz O, Vidal‐Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID‐19 vaccination. J Eur Acad Dermatol Venereol. 2021.
Piccolo V, Bassi A, Argenziano G, et al. BNT162b2 mRNA COVID‐19 vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol. 2021.
Freeman EE, McMahon DE, Lipoff JB, et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486–92.
Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–29.
Galván Casas C, Catala A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–7.
McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86(1):113–21.
Woo EJ, Mba-Jonas A, Dimova RB, et al. Association of receipt of the Ad26. COV2. S COVID-19 vaccine with presumptive Guillain-Barré syndrome, February-July 2021. JAMA. 2021;326(16):1606–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ms Falotico, Mr Desai, Mr Shah, and Dr Ricardo have no conflicts of interest. Dr Lipner has served as a consultant for Ortho Dermatologics, Verrica, and Hoth Therapeutics, BelleTorus Corporation, and Hexima.
Consent to participate/publish
Not applicable.
Funding sources
There was no funding for this study.
Data availability
All data generated or analyzed during this study are included in this published article. Further enquiries can be directed to the corresponding author.
Author contributions
All authors contributed substantially to this manuscript. Study concept and design: SRL. Acquisition of data: JMF, ADD, AS, SRL. Analysis and interpretation of data: JMF, ADD, AS, JWR, SRL. Drafting of the manuscript: JMF. Critical revision of the manuscript for important intellectual content: SRL. Study supervision: SRL.
IRB approval status
Not applicable.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Falotico, J.M., Desai, A.D., Shah, A. et al. Curbing COVID-19 Vaccine Hesitancy from a Dermatological Standpoint: Analysis of Cutaneous Reactions in the Vaccine Adverse Event Reporting System (VAERS) Database. Am J Clin Dermatol 23, 729–737 (2022). https://doi.org/10.1007/s40257-022-00715-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00715-x