Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin condition that can have tremendous impact on quality of life for affected children and adults. First-line therapy for acute management of AD includes topical therapies such as corticosteroids, calcineurin inhibitors, and, more recently, the phosphodiesterase inhibitor crisaborole. Topical agents have remained the mainstay therapy for decades; however, there has been a longstanding need for topical therapies with high efficacy and low risk of adverse effects with long-term use. Given the ongoing advances in understanding the pathogenesis of AD, there are novel targets for pharmacological intervention. We are now in an unprecedented time with more than 40 topical treatments in the pipeline for AD in addition to many developments and treatments on the horizon. This review summarizes selected therapeutic topical agents in later phases of development that target various aspects in the pathogenesis of AD such as Janus kinase inhibition (ruxolitinib and delgocitinib), phosphodiesterase-4 inhibition (roflumilast and difamilast), aryl hydrocarbon modulation (tapinarof), and modulation of the microbiome. We also review novel targeted therapies that are in early phase clinical trials, including AMTX-100, BEN-2293, and PRN473. Preliminary findings on efficacy and tolerability of most of these agents are promising, but further studies are warranted to evaluate the long-term safety and efficacy of these novel agents against the current standard of care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Topical ruxolitinib cream, a Janus kinase 1 (JAK1)/JAK2 inhibitor, has been approved for mild-to-moderate atopic dermatitis in patients aged 12+ years, based on safety and efficacy data, while other formulations of JAK inhibitors are under study for chronic hand eczema and atopic dermatitis. |
In addition to crisaborole, topical phosphodiesterase inhibitors roflumilast cream and difamilast ointment are therapeutic agents that have shown efficacy and tolerability in trials for treating mild-to-moderate atopic dermatitis in patients as young as 2 years old. |
Tapinarof is a novel, aryl hydrocarbon receptor-modulating agent that has reached phase III trials for the study of moderate-to-severe atopic dermatitis. |
1 Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease, affecting an estimated 16.5 million adults and 9.6 million children in the United States (US) [1, 2]. This chronic condition often begins in the first few years of life and may persist into adulthood; however, adolescent and adult-onset disease are increasingly recognized. The pathogenesis of AD is complex but involves a dysregulated immune system and a compromised skin barrier [3]. Mainstay therapy for acute management includes topical corticosteroids (TCSs) or non-steroidal alternatives including topical calcineurin inhibitors (TCIs) and phosphodiesterase inhibitors, such as crisaborole ointment [4].
Since the 1950s, TCSs have remained the standard of care due to their ability to reduce skin inflammation and pruritus. However, long-term use may cause localized skin atrophy, telangiectasia, striae, perioral dermatitis, and acne. With extensive use, there is also the rare risk of systemic absorption, which may lead to suppression of the hypothalamic-pituitary-adrenal axis, adversely affecting the linear growth and bone density of children and adults, respectively. As a result, patients and caregivers often underutilize TCSs due to fear of side effects, termed “steroid phobia” [5, 6].
TCIs have been approved (tacrolimus and pimecrolimus in 2000 and 2001, respectively) for short-term or intermittent use in patients who have previously failed on, or have contraindications to, TCSs. These second-line agents act as immunosuppressants and inhibit T cell activation, decreasing expression of pro-inflammatory cytokines. Tacrolimus 0.03% and 0.1% ointments are approved for adults and 0.03% for children aged 2–15 years with moderate-to-severe AD. Pimecrolimus 1% cream is indicated for non-immunocompromised adults and children aged ≥ 2 years with mild-to-moderate AD. Although these TCIs do not carry the risk of skin atrophy, they can cause local adverse effects of skin burning and irritation. In addition, a black box warning was added by the Food and Drug Administration (FDA) in 2006 regarding the hypothetical risk that TCIs may increase long-term cancer risk in individuals with AD; however, findings from multiple studies have not shown increased risks with their use [7, 8].
There were few innovations in topical therapy for AD in the past decade, but in 2016, a novel phosphodiesterase inhibitor, crisaborole 2% ointment, received FDA approval for adults and children aged ≥ 2 years with mild-to-moderate AD [9]. Evidence has demonstrated that patients with AD have elevated leukocyte phosphodiesterase activity, which may impact histamine and cytokine release [10]. Although the exact mechanism of action of crisaborole remains unclear, the inhibition of phosphodiesterase is thought to play a role in decreasing the skin inflammation and pruritus associated with AD. In 2020, the indicated age range was expanded to patients as young as 3 months of age, making crisaborole the first and only non-steroidal topical approved for this population in the US [11].
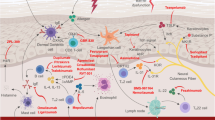
Over the years, there have been successful efforts to better understand the pathogenesis of AD. As such, an increasing number of therapeutic targets have been investigated. The different selected therapeutics discussed herein are topical therapies in early and late phases of clinical trials that target a variety of mechanisms involved in the pathogenesis of AD (Table 1).
2 JAK Inhibitors
The Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway plays an influential role in the pathogenesis of AD [12]. Several key cytokines, such as interleukin (IL)-4, IL-5, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin (TSLP) activate the JAK-STAT pathway to recruit immune cells, keratinocytes, and peripheral sensory neurons involved in propagating inflammation and itch [13]. AD is associated with increased signaling through all four JAKs [JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2)], unlike other autoimmune conditions such as psoriasis or alopecia areata, in which likely one predominant JAK pathway is dysregulated [14]. JAK inhibitors (JAKi) are small molecules that can either target specific JAK receptors, a combination of JAK receptors, or all four of the human JAKs (JAK1, JAK2, JAK3, and TYK2). A selective topical JAKi currently in clinical development is SHR0302 (JAK1, Reistone Biopharma). Dual topical JAKi include ruxolitinib (JAK1/JAK2, Incyte), brepocitinib (JAK1/TYK2, Pfizer), and ATI-1777 (JAK1/JAK3, Aclaris). Non-selective topical pan-JAKi approved for AD, or in clinical development, include delgocitinib (Japan Tobacco/LEO), cerdulatinib (RVT/DMVT502, Dermavant), jaktinib (Suzhou Zeigen Biopharma), and CEE321 (Novartis) [15]. In this article, we will review the two topical JAKi that have completed randomized, double-blind, vehicle-controlled, phase III clinical trials and are now on the market for treating AD: topical ruxolitinib cream (available in the US) and topical delgocitinib ointment (available in Japan).
2.1 Topical Ruxolitinib Cream
Topical ruxolitinib cream (Opzelura™), a topical JAK1/JAK2i, is a promising new topical therapy for AD. It gained US FDA approval for AD in September 2021 for short-term and non-continuous chronic treatment of mild-to-moderate AD in non-immunocompromised patients aged ≥ 12 years [16]. The Topical Ruxolitinib Evaluation in AD (TRuE-AD) clinical trial program consisted of phase III, randomized, double-blinded studies that investigated 1249 patients ≥ 12 years old with mild-to-moderate AD [Investigator's Global Assessment (IGA) score of 2–3] with a body surface area (BSA) of 3–20% (excluding scalp). Participants were randomly assigned in a 2:2:1 ratio of 0.75% ruxolitinib, 1.5% ruxolitinib, or vehicle for 8 weeks. The treatment was studied in two parallel studies, TRuE-AD1 and TRuE-AD2, which found similar successful results; more patients achieved the primary endpoint of an IGA score of 0–1 and ≥ 2-grade improvement from baseline at week 8 when using either 0.75% ruxolitinib (50.0% TRuE-AD1, 39.0% TRuE-AD2) or 1.5% (53.8%, 51.3%) compared with placebo (15.1%, 7.6%) [17]. Ruxolitinib cream also successfully reduced itch associated with AD. Patients achieved a significant decrease in itch, measured as a ≥ 4-point reduction in itch numerical rating scale score (NRS4) from baseline to week 8: 0.75% ruxolitinib (40.4% TRuE-AD1, 42.7% TRuE-AD2) and 1.5% (52.2%, 50.7%) versus vehicle (15.4%, 16.3%). This reduction in itch also occurred exceedingly fast, with data showing that on day 2 (~ 36 h after first application), the proportion of patients achieving itch NRS4 was higher with 1.5% ruxolitinib (11.6% TRuE-AD1, 10.8% TRuE-AD2) versus vehicle (2.9% TRuE-AD1, P = 0.048; 1.3% TRuE-AD2, P = 0.015). Aside from the improvement in itch, ruxolitinib cream was well-tolerated and showed an unremarkable safety profile for both strengths versus vehicle. The most common treatment-emergent (TE) adverse event (AE) was nasopharyngitis, observed primarily with 0.75% ruxolitinib [n = 15 (3.0%) vs n = 13 (2.6%) with 1.5% ruxolitinib and n = 2 (0.8%) with vehicle]. Although nasopharyngitis did not occur in the majority of patients, it was significantly higher in both ruxolitinib groups compared with the placebo group [17]. Of note, pharmacokinetic data studying maximal use of topical ruxolitinib demonstrated that serum levels in some individuals with extensive eczema BSA approached levels similar to that of low-dose oral ruxolitinib. However, studies with the approved topical 1.5% ointment used in patients with up to 20% BSA did not show evidence of accumulation nor hematologic changes of clinical significance over time [18].
Two sub-analyses of the combined TRuE-AD data were carried out to further study the impact of ruxolitinib cream on itch and its efficacy and safety in the adolescent population. The first sub-analysis established that the mean itch score decreased with ruxolitinib as early as day 1, even earlier than the original data suggested. The time required to see a significant improvement in itch was much faster in patients using ruxolitinib than placebo; patients with a baseline itch score of ≥ 2 applying the 0.75% and 1.5% ruxolitinib creams reported reaching a 2- to 3-point change in itch score from baseline in 5 and 4 days, respectively, compared with 17 days in the placebo group [19]. The second sub-analysis evaluated the TRuE-AD data in patients between the ages of 12 and 17 years and found that efficacy in the adolescent population was similar to that in adults, with achievement of ≥ 90% improvement in Eczema Area and Severity Index score (EASI-90) in 41.5% of adolescents taking 0.75% ruxolitinib cream, 39.1% for 1.5%, and 7.0% for placebo [20]. The safety in the adolescent population was comparable to the overall TRuE-AD patient population, with no treatment discontinuation due to AEs. To date, topical ruxolitinib has no published data of studies in patients below the age of 12.
2.2 Topical Delgocitinib Ointment
Another new topical JAKi is delgocitinib ointment, a topical pan-JAKi (JAK1, JAK2, JAK3, TYK2) that has been on the market in Japan for both children and adults with AD since January 2020 [21]. The initial phase III study included Japanese patients aged ≥ 16 years with modified Eczema Area and Severity Index (mEASI) scores of ≥ 10 (excluding head/neck), IGA scores of 3 (moderate) or 4 (severe), and BSA between 10% and 30%. The randomized, double-blind, phase III trial assigned patients in a 2:1 ratio to delgocitinib 0.5% ointment or vehicle ointment twice daily (maximum dose per application, 5 g) for a period of 4 weeks. There was a significant improvement in IGA and mEASI scores with delgocitinib; 26.4% (28/106) of patients in the delgocitinib group reached mEASI-75 compared to 5.8% (3/52) in the vehicle group (P < 0.01) [21]. A 52-week extension with 506 patients showed no significant long-term effects associated with extended use of this topical JAKi [22]. Overall, AEs were reported in 69.0% of patients, most being mild and unrelated to therapy. The most common AE was nasopharyngitis (25.9%), similar to the findings in the 4-week ruxolitinib study, followed by contact dermatitis (4.5%). Treatment-related (TR) AEs were reported in 78 patients (15.4%); the most common being application site events such as application site folliculitis (2.4%) and acne (2.2%), suggesting the lack of new AEs that develop with extended use [22].
The pediatric phase III clinical trial utilized a comparable methodology to the adult population albeit in Japanese patients between 2 and 15 years of age with a mEASI score ≥ 5, an IGA score ≥ 2 (mild), and BSA between 5% and 30% [23]. Participants were randomized in a 1:1 ratio to delgocitinib 0.25% ointment or vehicle for 4 weeks. The decision to study the 0.25% ointment was based on the phase II, randomized, double-blinded, 4-week study, in which pediatric patients were assigned 1:1:1 topical delgocitinib 0.25% ointment (n = 35), 0.50% ointment (n = 35), or vehicle (n = 35), which demonstrated greater numerical improvement effects for 0.5% delgocitinib ointment than for 0.25% delgocitinib ointment, but no significant differences [24]. Results from the phase III pediatric study demonstrated similar success to that of the adult population, with the least-squares mean percentage changes from baseline in the mEASI score being −39.3% in the delgocitinib group and + 10.9% in the vehicle group at end of treatment (EOT). An mEASI-75 was achieved by 37.7% of patients in the delgocitinib group compared with 4.4% in the vehicle group. Again, no new safety concerns emerged with delgocitinib ointment in pediatric patients with AD. Age was stratified into ranges of 2–6, 7–11, and 12–15 years. Systemic exposure to delgocitinib was low in all age groups of the present study, consistent with adult studies, indicating that delgocitinib ointment seems unlikely to pose increased risk of systemic infections regardless of age [24].
2.3 Topical Delgocitinib Cream
The cream formulation of delgocitinib remains under investigational therapy for chronic hand eczema (CHE). A phase IIa study randomized participants (n = 258) to delgocitinib cream twice daily for 16 weeks in four different doses (1 mg/g, 3 mg/g, 8 mg/g, 20 mg/g) or vehicle. By week 16, almost 40% of patients, regardless of dose, was clear or almost clear of symptoms based on IGA-CHE assessment [25]. The phase IIb study observed a statistically significant response for participants receiving delgocitinib 8 mg/g and 20 mg/g, suggesting a dose-dependent response [26]. Based on these results, LEO Pharma initiated the first phase III clinical trial with delgocitinib cream in May 2021. The phase III study intends to evaluate the efficacy and safety of twice-daily delgocitinib cream 20 mg/g, compared with vehicle, for 16-weeks in patients aged > 18 years with moderate-to-severe CHE (IGA ≥ 2) and an inadequate response to TCSs within 1 year of screening [27].
3 PDE4 Inhibitors
Another inflammatory AD pathway targeted by new topical therapies involves phosphodiesterase-4 (PDE4). PDE4 is an intracellular enzyme mainly present in immune, epithelial, and brain cells that regulates inflammation and epithelial integrity by degrading cyclic adenosine monophosphate [28]. PDE4 inhibition mediates inflammatory cytokines and has been utilized in various forms for respiratory diseases, cutaneous psoriasis, psoriatic arthritis, and AD [29, 30]. Crisaborole 2% ointment, a PDE4 inhibitor, received an expanded age indication by the FDA for AD down to patients aged ≥ 3 months [31]. There are several new topical PDE4 inhibitors that have been in development: lotamilast (RVT-501/E6005, Dermavant), difamilast (OPA-15406/MM36, Otsuka), DRM02 (Dermira), LEO 29102 (LEO Pharma), roflumilast (AstraZeneca), Hemay808 (Tianjin Hemay Pharmaceutical), and PF-07038124 (Pfizer) [16]. In this article, the PDE4 inhibitors we will focus on are topical roflumilast cream and topical difamilast ointment.
3.1 Topical Roflumilast Cream
Roflumilast cream completed phase III studies for plaque psoriasis, but its clinical investigation for AD is not far behind. A phase II, proof-of-concept study for roflumilast cream involved a small number of AD patients (n = 136) with 1.5–35% BSA, validated IGA (vIGA) 2–3, and EASI score ≥ 5 randomized to roflumilast 0.15%, 0.05%, or vehicle once daily for 4 weeks. The primary efficacy endpoint was absolute change from baseline in EASI score at week 4. Results at EOT showed a trend towards this endpoint but did not reach statistical significance, possibly due to the small sample size. Nevertheless, other efficacy endpoints for roflumilast cream 0.15% reached statistical significance at week 4, such as 72.3% EASI improvement and > 50% of patients achieving clear or almost clear skin on vIGA-AD. This study also showed that roflumilast cream was well-tolerated, with a low rate of application site reactions [32]. Despite the lack of statistical significance in reaching the primary endpoint, the favorable safety profile and encouraging efficacy results warranted further investigation of roflumilast cream for the treatment of AD in Arcutis’s phase III trial. The “INterventional Trial EvaluatinG roflUMilast cream for the treatmENt of aTopic dermatitis”, or INTEGUMENT, is a set of double-blind, vehicle-controlled trials in which roflumilast cream or vehicle is applied once daily for 4 weeks to patients with mild-to-moderate AD involving ≥ 3% BSA. The parallel INTEGUMENT-1 and INTEGUMENT-2 trials investigating roflumilast cream 0.15% in patients ≥ 6 years of age were initiated in February 2021 [33, 34]. INTEGUMENT-PED, the clinical trial investigating the lower dose of roflumilast 0.05% in pediatric patients between the ages of 2 and 5 years old, was initiated in April 2021 [35]. In these phase III trials, participants are randomized 2:1 to roflumilast cream (0.15% for ages ≥ 6 years or 0.05% for ages < 6 years) or vehicle. The primary endpoint is defined as a vIGA-AD score of ‘clear’ or ‘almost clear’ plus a 2-grade improvement from baseline at week 4.
3.2 Topical Difamilast Ointment
Difamilast ointment completed phase III trials in Japan in both the adult and pediatric populations, and received manufacturing and marketing approval for patients aged ≥ 2 years [36,37,38]. The adult study was a double-blind, vehicle-controlled trial investigating patients aged 15–70 years with a history of AD for at least 3 years, an IGA score of 2 (mild) or 3 (moderate), and BSA of 5–40% (excluding scalp) assigned in a 1:1 ratio to either difamilast 1% (n = 182) or vehicle (n = 182) ointment twice daily for 4 weeks [36]. The percentage of patients that reached the primary endpoint of IGA 0 or 1, with improvement by ≥ 2 grades, at week 4 was significantly higher in the 1% difamilast group (38.46%) than in the vehicle group (12.64%) (P < 0.0001). EASI-50 at EOT was 58.24% for 1% difamilast and 25.82% for vehicle (P < 0.0001); EASI-75 was 42.86% and 13.19%, respectively (P < 0.0001); and EASI-90 was 24.73% and 5.49%, respectively (P < 0.0001). The least square means change in verbal rating score for pruritus at week 4 was −0.65 in the 1% difamilast group and −0.04 in the vehicle group (P < 0.0001). The number of patients who discontinued treatment due to TEAEs was seven (3.8%) in the 1% difamilast group and 21 (11.5%) in the vehicle group, with the most common TEAE leading to discontinuation being worsening AD [7/182 (3.8%) with 1% difamilast vs. 17/182 (9.3%) with vehicle] [36]. The pediatric study followed thereafter as a double-blind, vehicle-controlled trial investigating patients aged 2–14 years with an IGA of 2 (mild) or 3 (moderate) and BSA of 5–40% (excluding scalp) assigned in a 1:1:1 ratio to either difamilast 0.3% ointment (n = 83), 1% (n = 85), or vehicle (n = 83) twice daily for 4 weeks [37]. The percentage of patients that reached the primary endpoint was 44.6%, 47.1%, and 18.1% in the difamilast 0.3%, 1%, and vehicle groups, respectively. Both difamilast groups (0.3% and 1%) demonstrated significantly higher success rates in IGA score compared with vehicle EOT (P < 0.001). EASI-50 at week 4 for difamilast 0.3%, 1%, and vehicle was 69.9%, 68.2%, and 31.3%, respectively; EASI-75 was 43.4%, 57.7%, and 18.1%; EASI-90 was 32.5%, 41.2%, and 7.2%.
4 Tapinarof Cream
Studies have demonstrated that the innate immune system plays a role in the early phase of AD [39]. Tapinarof (GSK2894512 cream, WBI-1001, or Benvitimod™) is a novel small-molecule topical therapeutic aryl hydrocarbon receptor (AHR) modulator currently in late-stage clinical trials for the treatment of both psoriasis and AD [40]. It originated as a naturally derived small molecule produced by bacterial symbionts [41]. Tapinarof’s mechanism of action in AD is thought to be through AHR activation, inhibiting IL-4/IL-13-mediated signal transducer and activator of transcription 6 (STAT6) activation and increasing expression of filaggrin (FLG), loricrin (LOR), and involucrin (IVL), which are downregulated in AD and associated with barrier dysfunction [40, 42]. Results from a recent phase II, double-blind, vehicle-controlled, randomized, six-arm trial (1:1:1:1:1:1) in patients aged 12–65 years, with BSA 5–35% and an IGA score ≥ 3 (moderate) at baseline, demonstrated that topical application of the natural AHR agonist tapinarof is efficacious and well-tolerated in patients with AD [43, 44]. The rates of treatment success with tapinarof cream at week 12, defined as an IGA score of clear or almost clear (0 or 1), were 53% (1% twice daily, n = 40), 46% (1% once daily, n = 41), 37% (0.5% twice daily, n = 43), 34% (0.5% once daily, n = 41), 24% (vehicle twice daily, n = 42), and 28% (vehicle once daily, n = 40). Notably, treatment success was maintained for 4 weeks after EOT. Dermavant Sciences initiated a phase III study in August 2021 to study tapinarof treatment for moderate-to-severe AD in children and adults ages ≥ 2 years [45]. This study is an 8-week double-blind, vehicle-controlled treatment study in which participants will be randomized to 1% tapinarof cream or vehicle once daily for 8 weeks. At the end of the 8-week study treatment, subjects may enroll in an open-label, long-term extension for an additional 48 weeks of treatment. These studies are ongoing.
5 Microbial Interventions
In recent years, an increasing number of studies have identified the microbiome as a key player in the pathogenesis of AD, with microorganisms functioning as modulators of both the innate and adaptive immune system [46]. Several studies have demonstrated that the skin of AD patients is characterized not only by a less diverse microbial landscape, but also with a higher rate of Staphylococcus aureus colonization and reduced commensal organisms (Streptococcus, Corynebacterium, Cutibacterium, and Proteobacteria) [47, 48]. Interestingly, the degree of microbiome diversity seems to correlate with the severity of AD [49]. In this article, we will review the existing data for the topical microbial therapeutics: Forte B-401 and Staphylococcus hominis A9 (ShA9).
Forte B-401 is a topical agent that consists of three therapeutic strains of the commensal gram-negative bacteria Roseomonas mucosa. Preclinical data demonstrate the ability of FB-401 to drive tissue repair and anti-inflammation as well as suppress potentially harmful bacteria like S. aureus. In a phase I/IIa, open-label study, 30 patients (n = 10 adults aged > 18 years, n = 20 children aged 3–16) were treated topically with FB-401 for 16 weeks (twice weekly for 12 weeks, then every other day for the final 4 weeks). Among the adult patients, 60% showed 50% reduction in the Scoring of AD (SCOR-AD). Equally important, 90% of the pediatric patients achieved EASI-50, and 30% achieved EASI-90. Moreover, treatment was associated with improvements in trans-epidermal water loss (14% reduction), pruritus (61% reduction), and topical steroid requirement (68% reduction). A modest shift in the skin microbiota diversity was also observed with a reduced S. aureus burden and increased presence of R. mucosa. These improvements persisted for up to 8 months post-treatment with no evidence of AEs or treatment complications [50]. Given these promising early results, Forte initiated a phase II, randomized, double-blind, 16-week, clinical trial consisting of 154 participants aged ≥ 2 years with mild-to-moderate AD. However, the study failed to meet statistical significance as only 58% of participants on FB-401 achieved EASI-50 compared to 60% of participants on placebo (P = 0.7567) [51].
An alternative microbial therapeutic agent under investigation is ShA9, a bacterium isolated from healthy human skin that has been shown to kill S. aureus and inhibit production of its inflammatory toxins in mouse studies. In a phase I/II trial completed in 2019, ShA9 or vehicle was applied topically twice daily for 7 days to the ventral arms of 54 participants with moderate-to-severe AD with positive S. aureus colonization. Results demonstrated that ShA9 reduced colony-forming units of S. aureus and improved EASI and SCORAD scores at these localized sites [52]. Current evidence supports the potential use of microbiome-based therapies, but these studies are still in early phase clinical trials.
6 Early Novel Targeted Agents
To date, there are more than 40 topical treatments in the pipeline for AD; however, this review will only discuss the following novel targeted agents that are in early phase clinical trials: AMTX-100 (NCT04313400), BEN-2293 (NCT04737304), and PRN473 (NCT04992546). AMTX-100 CF is formulated as a topical cream consisting of a novel 28-amino acid synthetic polypeptide (AMTX-100) bioengineered from human fibroblast growth factor-4 (FGF-4) and the nuclear localization sequence of nuclear factor kappa B(NF-kB). AMTX-100 is also available in various formulations (orals, injectables, eye drops and sprays) that are currently in pre-clinical studies to treat a wide range of inflammatory and autoimmune diseases, such as rosacea, psoriasis, rheumatoid arthritis, and uveitis. This product works intracellularly via the importin α/β complex to competitively inhibit transcription factors [i.e., NF-κB, nuclear factor of activated T cells (NFAT), activator protein 1 (AP-1), and STAT1], thereby reducing production of pro-inflammatory cytokines and chemokines. In pre-clinical animal studies, AMTX-100 has demonstrated therapeutic potential in addressing the itch and inflammation in mild-to-moderate AD. An ongoing phase I/II clinical trial with an estimated completion date in early 2023 is currently investigating the maximum tolerable dose based on maximum BSA percentage treated and the efficacy of various concentrations of AMTX-100 CF in 145 adult patients treated with AD [53].
BEN-2293 is a selective small-molecule pan-tropomyosin receptor kinase (pan-TRK) antagonist delivered topically. TRK is a family of high affinity nerve growth factors (TRKA, TRKB, TRKC) present in the human epidermis, dermis, and dorsal root ganglion that bind neurotrophins, which have been deemed to exacerbate skin inflammation [54]. There is no published literature on BEN-2293 to date, but BEN-2293 is currently in phase I/II clinical trials for 130 adult patients with mild-to-moderate AD, which is based similarly on the phase IIb clinical trial investigating topical TRKA inhibitor CT327 for treating pruritus in psoriasis [55].
PRN473 topical is a reversible, covalent Bruton tyrosine kinase (BTK) inhibitor. BTK is exclusively expressed in B cells and innate immune cells and plays a key role in B-cell receptors, Fc receptors, and other innate immune cell signaling pathways. PRN473 is also available in an oral and central nervous system formulation that is in phase III clinical trials to treat systemic diseases (pemphigus, immune thrombocytopenia, IgG4-related disease) and multiple sclerosis, respectively. Preclinical studies in rodents demonstrated that topical PRN473 can inhibit both IgG-mediated passive Arthus reaction and cutaneous IgE-mediated anaphylaxis [56]. There is an active phase II clinical trial for this novel target, for up to 40 patients with mild-to-moderate AD.
7 Conclusion
The aim of developing targeted therapy for AD is to provide control of inflammation and pruritus with improvement of barrier function while minimizing AEs seen with the use of TCSs and TCIs. By elucidating the complex pathogenesis of AD, additional targets for therapy can be developed. Many patients with AD require lifelong treatment. Thus, there is an unmet need for topical therapies with high efficacy and low risk of AEs with long-term use. Non-steroidal topical therapies that work regionally with minimal systemic effects may deliver effective disease control, without concerns of skin atrophy, telangiectasia, striae, or perioral dermatitis associated with TCSs. These novel treatments are part of the frontier for expanding our armamentarium to bring relief to a wider range of AD patients with varying needs.
References
Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–90. https://doi.org/10.1016/j.jid.2018.08.028.
Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. https://doi.org/10.1038/jid.2010.251.
Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–94. https://doi.org/10.1056/nejmra074081.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. https://doi.org/10.1016/j.jaad.2014.03.023
Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142(5):931–6. https://doi.org/10.1046/j.1365-2133.2000.03473.x.
Niemeier V, Kupfer J, Schill WB, Gieler U. Atopic dermatitis—topical therapy: do patients apply much too little? J Dermatol Treat. 2005;16(2):95–101. https://doi.org/10.1080/09546630510027895.
Asgari MM, Tsai AL, Avalos L, Sokil M, Quesenberry CP Jr. Association between topical calcineurin inhibitor use and keratinocyte carcinoma risk among adults with atopic dermatitis. JAMA Dermatol. 2020;156(10):1066–73. https://doi.org/10.1001/jamadermatol.2020.2240.
Paller AS, Fölster-Holst R, Chen SC, Diepgen TL, Elmets C, Margolis DJ, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83(2):375–81. https://doi.org/10.1016/j.jaad.2020.03.075.
Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494-503.e6. https://doi.org/10.1016/j.jaad.2016.05.046.
Grewe SR, Chan SC, Hanifin JM. Elevated leukocyte cyclic AMP-phosphodiesterase in atopic disease: a possible mechanism for cyclic AMP-agonist hyporesponsiveness. J Allergy Clin Immunol. 1982;70(6):452–7. https://doi.org/10.1016/0091-6749(82)90008-2.
Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21(2):275–84. https://doi.org/10.1007/s40257-020-00510-6.
Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3): e24137. https://doi.org/10.4161/jkst.24137.
Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927–40. https://doi.org/10.1016/j.jaci.2021.08.009.
O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2(0 2):ii111–ii115. https://doi.org/10.1136/annrheumdis-2012-202576
Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease [published online ahead of print]. Nat Rev Drug Discov. 2021;1–20. https://doi.org/10.1038/s41573-021-00266-6
Prescribing information for opzelura (ruxolitinib) cream 1.5%; Revised 2021 Sept [cited 2022 Feb 13]; https://www.opzelura.com/prescribing-information.pdf
Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung D, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85(4):863–72. https://doi.org/10.1016/j.jaad.2021.04.085.
Gong X, Chen X, Kuligowski ME, Liu X, Liu X, Cimino E, et al. Pharmacokinetics of ruxolitinib in patients with atopic dermatitis treated with ruxolitinib cream: data from phase II and III studies. Am J Clin Dermatol. 2021;22(4):555–66. https://doi.org/10.1007/s40257-021-00610-x.
Blauvelt A, Szepietowski JC, Papp K, et al. Ruxolitinib cream rapidly decreases pruritus in atopic dermatitis: pooled results from two phase 3 studies. Presented at: AAD VMX 2021; April 23–26, 2021. Poster 26884.
Eichenfield LF, Simpson EL, Siegfried EC, et al. Efficacy and safety of ruxolitinib cream among adolescents with atopic dermatitis: pooled results from two phase 3 studies. Presented at: AAD VMX 2021; April 23–26, 2021. Poster 27633.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823–31. https://doi.org/10.1016/j.jaad.2019.12.015.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Murata R, Kaino H, et al. Long-term safety and efficacy of delgocitinib ointment, a topical janus kinase inhibitor, in adult patients with atopic dermatitis. J Dermatol. 2020;47(2):114–20. https://doi.org/10.1111/1346-8138.15173.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kabashima K, Oda M, et al. Delgocitinib ointment in pediatric patients with atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and a subsequent open-label, long-term study. J Am Acad Dermatol. 2021;85(4):854–62. https://doi.org/10.1016/j.jaad.2021.06.014.
Nakagawa H, Nemoto O, Igarashi A, Saeki H, Oda M, Kabashima K, et al. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis [published correction appears in J Allergy Clin Immunol. 2021 Oct;148(4):1088]. J Allergy Clin Immunol. 2019;144(6):1575–1583. https://doi.org/10.1016/j.jaci.2019.08.004
Worm M, Bauer A, Elsner P, Mahler V, Molin S, Nielsen TSS. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182(5):1103–10. https://doi.org/10.1111/bjd.18469.
Worm M, Thyssen JP, Schliemann S, Bauer A, Shi VY, Ehst B, et al. The topical pan-JAK inhibitor delgocitinib cream demonstrates dose response in a 16-week phase 2b trial in chronic hand eczema. D1T03.4A, EADV 2020 Virtual Congress, 29–31 Oct.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT04872101, Efficacy and safety of delgocitinib cream in adults with moderate to severe chronic hand eczema (DELTA 2); 2021 May 4 [cited 2022 Feb 13]; https://clinicaltrials.gov/ct2/show/NCT04872101
Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. https://doi.org/10.3389/fphar.2018.01048.
Schafer PH, Truzzi F, Parton A, Wu L, Kosek J, Zhang LH, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28(7):753–63. https://doi.org/10.1016/j.cellsig.2016.01.007.
Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. J Am Acad Dermatol. 2018;78(3):S43–52. https://doi.org/10.1016/j.jaad.2017.11.056.
Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, Effectiveness, and pharmacokinetics of crisaborole in infantsa aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1). Am J Clin Dermatol. 2020;21(2):275–84. https://doi.org/10.1007/s40257-020-00510-6.
Gooderham MJ, Kircik LH, Zirwas M, Lee M, Kempers SE, Draelos ZD, et al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in atopic dermatitis: phase 2 proof-of-concept study. Br J Dermatol. 2021;184:e56–e87. https://doi.org/10.1111/bjd.19722
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT04773587, Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis (INTEGUMENT-I); 2021 Feb 26 [cited 2022 Feb 13]; https://clinicaltrials.gov/ct2/show/NCT04773587?term=roflumilast&cond=atopic+dermatitis&draw=2&rank=3
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT04773600, Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis (INTEGUMENT-II); 2021 Feb 26 [cited 2022 Feb 13]; https://clinicaltrials.gov/ct2/show/NCT04773600?term=roflumilast&cond=atopic+dermatitis&draw=1&rank=4
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT04845620, Trial of PDE4 inhibition with roflumilast for the management of atopic dermatitis (INTEGUMENT-PED); 2021 April 15 [cited 2022 Feb 13]; https://clinicaltrials.gov/ct2/show/NCT04845620?term=roflumilast&cond=atopic+dermatitis&draw=1&rank=5
Saeki H, Ito K, Yokota D, Tsubouchi H. Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized double-blind vehicle-controlled trial. J Am Acad Dermatol. 2021;S0190–9622(21):02682–7. https://doi.org/10.1016/j.jaad.2021.10.027.
Saeki H, Baba N, Ito K, Yokota D, Tsubouchi H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial. Br J Dermatol. 2021. https://doi.org/10.1111/bjd.20655.
Otsuka Pharmaceutical Co., Ltd. [Internet]. Otsuka's moizerto® ointment granted approval in japan as a treatment for atopic dermatitis; 2021 Sept 27 [cited 2022 Feb 13]; https://www.otsuka.co.jp/en/company/newsreleases/2021/20210927_1.html
Chieosilapatham P, Kiatsurayanon C, Umehara Y, Trujillo-Paez JV, Peng G, Yue H, et al. Keratinocytes: innate immune cells in atopic dermatitis. Clin Exp Immunol. 2021;204(3):296–309. https://doi.org/10.1111/cei.13575.
Bissonnette R, Stein Gold L, Rubenstein DS, Tallman AM, Armstrong A. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84(4):1059–67. https://doi.org/10.1016/j.jaad.2020.10.085.
Furue M, Hashimoto-Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019;20(21):5424. https://doi.org/10.3390/ijms20215424.
Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: pathogenic implications in atopic dermatitis. Int J Mol Sci. 2020;21(15):5382. https://doi.org/10.3390/ijms21155382.
Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. 2019;80:89–98. https://doi.org/10.1016/j.jaad.2018.06.047.
Paller AS, Stein Gold L, Soung J, Tallman AM, Rubenstein DS, Gooderham M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84(3):632–8. https://doi.org/10.1016/j.jaad.2020.05.135.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Identifier NCT05014568, Tapinarof for the treatment of atopic dermatitis in children and adults; 2021 August 20 [cited 2022 Feb 13]; https://clinicaltrials.gov/ct2/show/NCT05014568
Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16(3):143–55. https://doi.org/10.1038/nrmicro.2017.157.
Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):26–35. https://doi.org/10.1016/j.jaci.2018.11.015.
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680. https://doi.org/10.1126/scitranslmed.aah4680
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–9. https://doi.org/10.1101/gr.131029.111.
Myles IA, Castillo CR, Barbian KD, Kanakabandi K, Virtaneva K, Fitzmeyer E, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12(560):eaaz8631. https://doi.org/10.1126/scitranslmed.aaz8631
Press release from Forte Bioscience: Forte Biosciences, Inc. announces first patient dosed in the clinical trial of FB-401 for the treatment of children and adults with atopic dermatitis: https://www.fortebiorx.com/investor-relations/news/news-details/2021/Clinical-Trial-of-FB-401-For-the-Treatment-of-Atopic-Dermatitis-Fails-to-Meet-Statistical-Significance/default.aspx
Nakatsuji T, Hata TR, Tong Y, Cheng JY, Shafiq F, Butcher AM, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27(4):700–9. https://doi.org/10.1038/s41591-021-01256-2.
Press release from Amytrx Therapeutics: Amytrx therapeutics emerges from stealth to develop novel therapies for inflammatory diseases with lead program AMTX-100 currently in clinical development for dermatologic indications: https://www.prnewswire.com/news-releases/amytrx-therapeutics-emerges-from-stealth-to-develop-novel-therapies-for-inflammatory-diseases-with-lead-program-amtx-100-currently-in-clinical-development-for-dermatologic-indications-301133549.html
Peters EM, Liezmann C, Spatz K, Daniltchenko M, Joachim R, Gimenez-Rivera A, et al. Nerve growth factor partially recovers inflamed skin from stress-induced worsening in allergic inflammation. J Invest Dermatol. 2011;131(3):735–43. https://doi.org/10.1038/jid.2010.317.
Roblin D, Yosipovitch G, Boyce B, Robinson J, Sandy J, Mainero V, et al. Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: results from experimental studies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm Venereol. 2015;95(5):542–8. https://doi.org/10.2340/00015555-2047.
Xing Y, Chu KA, Wadhwa J, Chen W, Zhu J, Bradshaw JM, et al. Preclinical mechanisms of topical PRN473, a bruton tyrosine kinase inhibitor, in immune-mediated skin disease models. Immunohorizons. 2021;5(7):581–9. https://doi.org/10.4049/immunohorizons.2100063.
Author information
Authors and Affiliations
Contributions
EK and JL performed the literature search and drafted the manuscript. LE and LM edited the manuscript.
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Ms. Laborada, Ms. Kleinman, and Dr. Metterle report no conflicts of interest. Dr. Eichenfield has served as a scientific adviser, consultant, and/or clinical study investigator for AbbVie, Almirall, Arena, Aslan, Arcutis, Arena, Dermavant, Eli Lilly, Forté, Galderma, Ichnos, Incyte, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, and Sanofi Genzyme.
Ethics approval
This study met the definition of institutional review board exempt research.
Consent to participate/publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kleinman, E., Laborada, J., Metterle, L. et al. What’s New in Topicals for Atopic Dermatitis?. Am J Clin Dermatol 23, 595–603 (2022). https://doi.org/10.1007/s40257-022-00712-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00712-0