Abstract
Background
Moderate‐to‐severe atopic dermatitis (AD) often requires long-term management with systemic therapies.
Objective
Our objective was to report the safety and efficacy of dupilumab treatment up to 4 years in adults with moderate-to-severe AD and efficacy in a subgroup of patients who transitioned from dupilumab once-weekly (qw) to administration every other week (q2w).
Methods
This interim analysis of the open-label extension study (NCT01949311) evaluated dupilumab 300 mg qw or q2w in adults previously enrolled in dupilumab trials for moderate-to-severe AD. Patients switched from qw to q2w following protocol amendment. The primary outcome was safety; efficacy was also assessed.
Results
Of 2677 patients enrolled and treated, 352 (13.1%) completed week 204 (end of efficacy assessments) and 202 (7.5%) completed safety follow-up through week 244. Self-reported compliance was 98.1%. Dupilumab’s safety profile was consistent with previous reports. Common treatment-emergent adverse events (≥5%) included nasopharyngitis, AD, upper respiratory tract infection, oral herpes, conjunctivitis, injection-site reaction, and headache. At week 204, mean ± standard deviation (SD) Eczema Area and Severity Index was 2.46 ± 3.98, and mean percent change from parent study baseline (PSBL) was −91.07%; mean ± SD Pruritus Numerical Rating Scale score was 2.10 ± 1.83, and mean percent change from PSBL was −68.74%. Efficacy was maintained in patients (n = 226) who transitioned from qw to q2w dosing. Limitations of this study included its open-label design, the lack of control arm, and smaller subsets of patients at later timepoints and receiving the approved q2w regimen.
Conclusion
These results support dupilumab as continuous long-term treatment for adults with moderate-to-severe AD; efficacy was sustained following transition from qw to q2w dosing.
Trial Registration ClinicalTrials.gov
NCT01949311.
Plain Language Summary
Atopic dermatitis is a chronic skin disease associated with inflamed skin and intense itching. People with moderate-to-severe atopic dermatitis often need long-term treatment, but many available treatments do not have demonstrated long-term safety data. In multiple clinical trials, dupilumab treatment resulted in significant improvements in signs and symptoms of atopic dermatitis. This study examined the safety and efficacy of up to 4 years of dupilumab treatment in adults with moderate-to-severe atopic dermatitis, and whether dupilumab continued to be effective in patients who switched from receiving treatment each week to treatment every other week. To address these questions, we collected data from adults who received 300 milligrams of dupilumab every week or every other week. In this study, safety findings were consistent with the known dupilumab safety profile. Patients' signs and symptoms were evaluated before and during treatment with evaluation tools including the Eczema Area and Severity Index (EASI), which indicates the extent and severity of disease, and the Pruritus Numerical Rating Scale (NRS), which indicates the intensity of itching. Reductions of 91% in EASI scores and 69% in Pruritus NRS scores showed that the improvement in signs and symptoms persisted for 204 weeks (almost 4 years) of treatment, and these effects were sustained following the switch from weekly treatment to the approved every other week treatment with dupilumab. The safety and efficacy data presented here support the use of dupilumab as a continuous, long-term treatment for up to 4 years for adults with moderate-to-severe atopic dermatitis.
AbstractSection Graphical abstract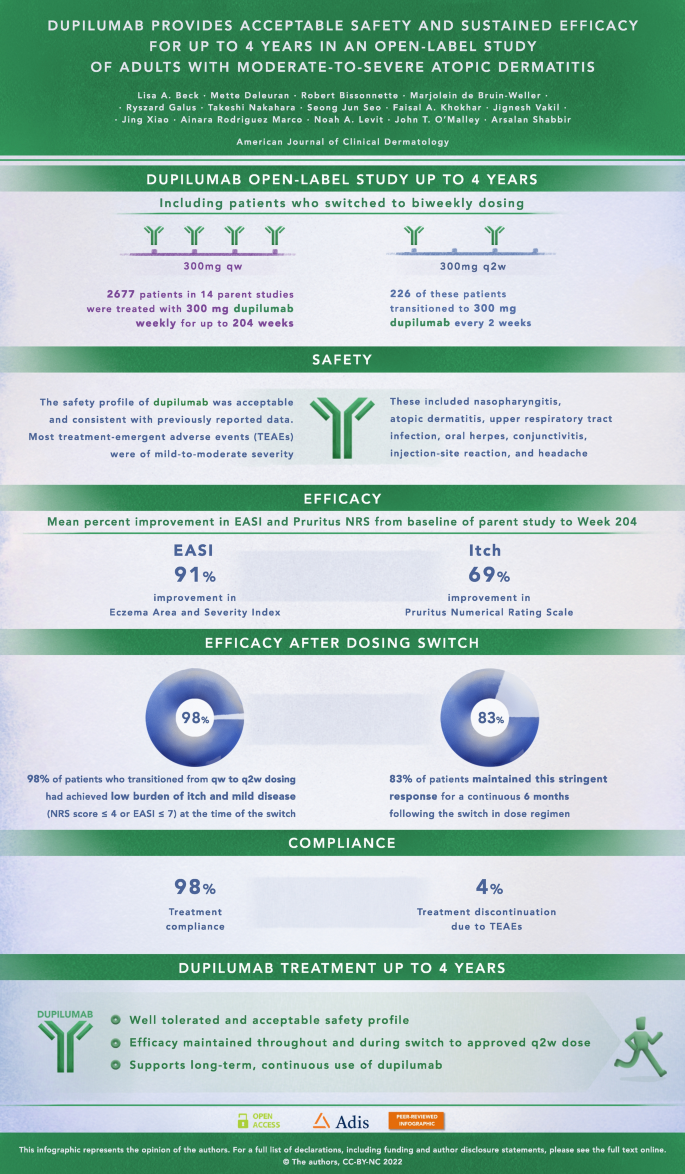
Video abstract: What is the long-term safety and efficacy profile of dupilumab in adults with moderate-to-severeatopic dermatitis for up to 4 years? (MP4 102515 KB)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.19342427. |
Dupilumab showed acceptable safety and sustained efficacy in adults with moderate-to-severe atopic dermatitis (AD) for up to 4 years. |
Safety data were consistent with 52-week controlled studies and with the known safety profile of dupilumab. |
Response to dupilumab was sustained following dose regimen change from 300 mg weekly to 300 mg every 2 weeks. |
The safety and efficacy data reported here support long-term, continuous dupilumab use in adults with moderate-to-severe AD. |
1 Introduction
Atopic dermatitis (AD) is a common chronic inflammatory systemic disease characterized by intense itching and recurrent eczematous lesions [1]. Management of moderate‐to‐severe AD often requires long-term treatment with systemic therapies; however, many available AD treatments are not recommended for long-term use because of safety concerns, and some newer investigational treatments lack long-term safety data [2]. For example, systemic corticosteroids (SCSs) are often used to treat severe AD exacerbations but are not recommended for treatment durations longer than 2 weeks; rebound flares are common following treatment cessation [2, 3]. The maximum recommended treatment duration for cyclosporine is 1 year in the USA and 2 years in Europe because of safety concerns; cyclosporine also requires close monitoring of vital signs and laboratory parameters to detect untoward changes in blood pressure and renal function during use [2, 4]. Other systemic immunosuppressants, including methotrexate, azathioprine, and mycophenolate mofetil, are commonly used off label to treat moderate-to-severe AD, but serious adverse events (AEs) have been reported [2]. An open-label study assessed the efficacy and safety of methotrexate and azathioprine in patients with moderate-to-severe AD up to 5 years, but the study sample size was small (N = 27), and drug continuation at 5 years was low because of AEs and lack of efficacy [5].
Dupilumab, a fully human VelocImmune®-derived [6, 7] monoclonal antibody that blocks the shared receptor subunit for interleukin (IL)-4 and IL-13, is approved for patients with type 2 inflammatory diseases, including AD, asthma, and chronic rhinosinusitis with nasal polyps [8, 9]. In multiple phase III clinical trials of dupilumab monotherapy or in combination with topical corticosteroids (TCSs), dupilumab provided rapid and sustained improvements in AD signs and symptoms and quality of life, with an acceptable safety profile [10,11,12,13,14,15].
Given that AD is a chronic relapsing disease characterized by flares and often requires continuous long-term treatment for stable disease control, analyses of long-term safety and efficacy data over time are critically important. Previous analyses of dupilumab treatment in a placebo-controlled study up to 52 weeks (LIBERTY AD CHRONOS) and an open-label extension study up to 3 years (LIBERTY AD OLE) have demonstrated acceptable safety and sustained efficacy in adults with moderate-to-severe AD [10, 16, 17]. Our objective in this report is twofold: (1) to describe an interim analysis of safety and efficacy results from the LIBERTY AD OLE study in adults with moderate-to-severe AD treated with dupilumab up to 4 years and (2) to describe efficacy results in a subgroup of patients (n = 226) who transitioned from dupilumab 300 mg weekly (qw) after at least 156 weeks of treatment to 300 mg every 2 weeks (q2w) and were followed for approximately 48 weeks (median 48.5 weeks; interquartile range 45.71–51.71) after the transition.
2 Methods
2.1 Study Design, Patients, and Treatment
The LIBERTY AD open-label extension (OLE) study (NCT01949311) is an ongoing phase III multicenter trial to assess the long-term safety and efficacy of dupilumab in adults with moderate-to-severe AD [18]. Two analyses (with data cutoff dates of April 11, 2016, and December 1, 2018) have been previously reported [16, 17]. Here, we report results with a cutoff date of March 19, 2021 (database lock April 28, 2021), which includes patients from approximately 550 sites in 28 countries in North America, Europe, and Asia-Pacific (Table S1 in the electronic supplementary material [ESM]).
The OLE enrolled adult patients who previously participated in dupilumab AD trials (phases I–III) [10, 12, 18,19,20,21,22,23,24,25,26]. Patients were ineligible if they had an AE related to dupilumab that led to treatment discontinuation or a serious AE related to dupilumab in the parent study. Details of enrollment criteria have been reported in greater detail previously [17].
Patients enrolled from the start of the study in October 2013 received a subcutaneous dose of dupilumab 200 mg qw (with a 400-mg loading dose). On June 12, 2014, the protocol was amended to a dose regimen of 300 mg qw based on results from a dose ranging study (NCT01859988). On November 12, 2019, the protocol was amended to a dose regimen of 300 mg q2w to align with the regimen approved by regulatory agencies [8, 9]. A complete list of protocol amendments is provided in Table 1.
Concomitant treatments for AD, including TCSs and topical calcineurin inhibitors (TCIs), were permitted, although use of SCSs and nonsteroidal systemic immunosuppressive medications (including phototherapy) as rescue medications required temporary discontinuation from the study treatment for the duration of rescue treatment and for five half-lives of the rescue treatment. Following Protocol Amendment 7 (June 2, 2017), patients were not required to temporarily discontinue study treatment following rescue treatment with SCSs. Originally, the planned study duration per patient was up to 3 years of dupilumab treatment or until regulatory approval of dupilumab in the patient’s enrollment country, whichever came first. The study duration was later amended to 5 years in Protocol Amendment 8 (January 8, 2018), allowing some patients to re-enter the study and resume dupilumab treatment at week 156 following a treatment interruption. Protocol amendment history has been reported in greater detail previously [17].
2.2 Ethics
This study was conducted in accordance with the Declaration of Helsinki, the International Council for Harmonisation guideline, good clinical practice, and local applicable regulatory requirements, including institutional review board approval. All patients provided written informed consent before any study procedures began.
2.3 Outcomes Assessed in This Analysis
The primary endpoint of the OLE was the incidence and rate (events per patient-year [PY]) of treatment-emergent AEs (TEAEs). Key secondary endpoints included the incidence and rate of serious TEAEs and AEs of special interest, proportion of patients with an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear), and proportion of patients with a ≥75% improvement (reduction) in the Eczema Area and Severity Index (EASI) from baseline of the parent study (EASI-75). Of note, IGA and EASI assessments at the end of treatment visit were removed from the protocol and subsequently restored during amendments 6 and 7, respectively. Additional secondary endpoints included change and percent change in EASI from baseline, proportion of patients with a ≥50% or ≥90% reduction in EASI (EASI-50 or EASI-90) from baseline of parent study, change and percent change in weekly average Pruritus Numerical Rating Scale (NRS) score, proportion of patients with a ≥3-point improvement (reduction) from baseline in weekly average Pruritus NRS score or with a score of 0, proportion of patients with a ≥4-point reduction from baseline in weekly average Pruritus NRS or a score of 0, and percentage of patients requiring rescue treatment. Post hoc outcomes included the proportion of patients with a ≥2-point improvement in IGA score from baseline. Additional post hoc outcomes assessed dupilumab efficacy before and after patients switched from qw to q2w dosing, including the proportion of patients with IGA 0 or 1, mean EASI, and mean weekly average Pruritus NRS score at baseline of q2w dosing and 48 weeks prior to and after this baseline. The proportion of patients who switched qw to q2w dosing with EASI ≤7 or Pruritus NRS score ≤4 who maintained a continuous well-controlled response for >24 weeks was also assessed.
2.4 Statistical Analysis
Analyses were performed on the safety analysis set, which comprised patients who received at least one dose of the study drug. Rescue medication use was manually adjudicated. Exposure-adjusted analyses for safety outcomes were calculated for the duration of study participation. Efficacy outcomes were assessed up to week 204 and included all observed data at each timepoint; missing values were not imputed. Efficacy data were only reported up to week 204 because of the small number of patients with efficacy data collected after this timepoint. Sensitivity analyses on patients who completed week 204 (or who withdrew but were enrolled in the study at least 204 weeks prior to the OLE study cutoff) were performed on some EASI and weekly average Pruritus NRS endpoints, using observed cohort and the last observation carried forward (LOCF; missing values imputed) approach. SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA), was used for all analyses. Because of the absence of a control arm in LIBERTY AD OLE, safety results from LIBERTY AD CHRONOS (NCT02260986) [10] are provided for comparison. The CHRONOS trial was selected for comparison because of its large sample size, 52-week duration, and mandatory use of concomitant TCSs.
3 Results
3.1 Patients
A total of 2827 adult patients were screened from 14 parent studies (Fig. 1). Screen failures (n = 149) included the following: consent withdrawn (n = 8), exclusion criteria met (n = 62), inclusion criteria not met (n = 48), lost to follow-up (n = 2), and other (n = 29). Of these, 2678 were enrolled and 2677 were included in the safety analysis set (one patient was enrolled but not treated). As of the cutoff date for this analysis, 2207 (82.4%) patients completed up to week 52, 1065 (39.8%) up to week 100, 557 (20.8%) up to week 148, 352 (13.1%) up to week 204, and 202 (7.5%) up to week 244. Of these, 1114 (41.6%) completed the study, 201 (7.5%) were continuing treatment, and 1362 (50.9%) had withdrawn from the study (Table 2, Fig. 1). The primary reason patients chose to withdraw from the study (810 [30.3%]) was study termination by sponsor following regulatory approval and commercial availability of dupilumab. A total of 238 (8.9%) patients withdrew from the study for reasons including relocation, desire for pregnancy, did not want to discontinue treatment for 12 weeks during the follow-up period, work/school conflict, and unspecified personal reasons. Few patients withdrew because of AEs (114 [4.3%]) or lack of efficacy (58 [2.2%]). Reasons for patient withdrawal due to AEs were not captured systematically as with a formally reported clinical safety event and thus are not summarized here. Self-reported compliance was 98.1%, and most (98.9%) patients reported ≥80% injection compliance.
Baseline demographics and disease characteristics have been reported previously [17]; no new patients were enrolled since the previous report. During the OLE treatment period, 53.3% of patients used TCSs, 16.3% used TCIs, 56% used TCSs or TCIs, and 13.6% used TCSs and TCIs. Few patients required rescue medication (47 [1.8%]); of these patients, 44 (1.6%) used systemic corticosteroids, and three (0.1%) used cyclosporine. The extent of either TCS, TCI, or both classes for rescue medication was not captured as part of the protocol.
Table 3 shows the mean duration of dupilumab exposure (300 mg qw or dupilumab 300 mg q2w) among patients who switched from qw to q2w. The overall duration of exposure to open-label dupilumab among this group of patients ranged from 182 to ≥260 weeks; mean ± standard deviation (SD)/median exposure: 241.7 ± 16.4/242.1 weeks. This cohort (n = 226) had an initial exposure duration of at least 3 years to dupilumab 300 mg qw. The duration of time patients spent on the qw dose ranged from 156 to <260 weeks, and the duration of time patients spent on the q2w dose regimen ranged from 4 to <76 weeks; mean ± SD/median qw exposure 195.0 ± 19.2/194.1 weeks; mean ± SD/median q2w exposure 46.7 ± 7.4/48.5 weeks. Of those who received the q2w regimen, 175 patients had exposure for 24 to <52 weeks, and 47 had exposure for >52 weeks.
3.2 Safety Assessment
A total of 14,569 TEAEs were reported during the OLE, with 2273 (84.9%) patients experiencing at least one TEAE (Table 4). The exposure-adjusted TEAE incidence rate was 256.86 number of events [nE]/100 PY. Most TEAEs were mild to moderate in severity, with 10.4 and 9.8% of patients experiencing at least one serious or severe TEAE, respectively. Treatment was discontinued because of TEAEs in 3.7% of patients. Serious TEAEs deemed related to study treatment occurred in 1.2% of patients, with each TEAE reported by Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) occurring in <0.1% of patients. Three deaths occurred during the OLE, all of which were deemed unrelated to study treatment. Circumstances of death were natural causes in an 88-year-old woman, unknown causes in a 60-year-old woman approximately 5 months after the final dose of study drug, and unknown causes in a 42-year-old man. The most common TEAEs (≥5% incidence; reported by PT) included nasopharyngitis, AD, upper respiratory tract infection, oral herpes, conjunctivitis, injection-site reaction, and headache. Incidences of the MedDRA high-level terms (HLTs) injection-site reactions and herpes viral infections were 9.8 and 12.8%, respectively. Skin infections (adjudicated PTs reported under the MedDRA system organ class “infections and infestations”) occurred in 9.3% of patients, including bacterial infections (3.2% of patients) and tinea infections (1.9% of patients), reported under their respective MedDRA HLTs. Eczema herpeticum (MeDRA PT) occurred in less than 1% of patients. The exposure-adjusted incidence rates for these TEAEs in the OLE at week 204 were generally lower than in the 300 mg qw arm in CHRONOS at 52 weeks (Table 4) [17].
Rates of serious TEAEs and serious TEAEs related to treatment were similar between patients in the OLE and patients receiving dupilumab in CHRONOS [17] (Table 4). In total, 383 serious TEAEs were reported in 278 patients (10.4%), and 38 serious TEAEs related to treatment were reported in 33 patients (1.2%). The exposure-adjusted incidence rate of serious TEAEs related to treatment in the OLE (0.58 number of patients [nP]/100 PY) was lower than that observed in the placebo arm in CHRONOS (1.06 nP/100 PY) and comparable with that observed in the dupilumab 300 mg qw arm in CHRONOS (0.68 nP/100 PY). The most common serious TEAEs (≥0.2% incidence; reported by MeDRA PT) included squamous cell carcinoma of the skin (0.14 nP/100 PY), osteoarthritis (0.16 nP/100 PY), AD (0.11 nP/100 PY), syncope (0.09 nP/100 PY), inguinal hernia (0.09 nP/100 PY), appendicitis (0.09 nP/100 PY), and ligament rupture (0.09 nP/100 PY). Skin infections were lower among patients who received dupilumab in either the OLE (4.69 nP/100 PY) or CHRONOS (7.87 nP/100 PY) compared with the placebo arm of CHRONOS (20.21 nP/100 PY).
Conjunctivitis (reported as a narrow cluster of MeDRA PTs: conjunctivitis, conjunctivitis allergic, conjunctivitis bacterial, conjunctivitis viral, and atopic keratoconjunctivitis) was reported in 20% of patients, with most cases being of mild (9.3%) or moderate (9.8%) severity as assessed by the investigator (Table 4). One case of serious conjunctivitis was reported but was deemed unrelated to the study drug. The exposure-adjusted incidence rate of conjunctivitis in the OLE (15.66 nE/100 PY) was lower than in the 300 mg qw arm at 52 weeks in CHRONOS (30.6 nE/100 PY). Most cases of conjunctivitis resolved during the study treatment period (775 of 888 cases; 87%). The most frequently used ophthalmic treatments included intraocular corticosteroids, antiallergy medications, and anti-infectives. Severe conjunctivitis occurred in 26 (1.0%) patients, and conjunctivitis leading to drug discontinuation occurred in 14 (0.5%) patients. Most conjunctivitis incidents occurred early in the study, and incidence decreased throughout the course of treatment (Table 4).
3.3 Efficacy
AD signs and symptoms improved throughout the OLE, with EASI and weekly average Pruritus NRS scores improving rapidly during the first several weeks, followed by incremental improvements throughout the remainder of the treatment period (Table 5, Figs. 2, 3, 4). A slight transient increase in mean EASI and mean weekly average Pruritus NRS score was observed at week 156 (visit 31), corresponding with patient re-entry to the study following treatment interruption (Figs. 3 and 4). Sensitivity analyses conducted to assess any bias on treatment outcomes because of patient withdrawal were consistent with results from all observed patients (Table 5, Figs. 3, 4).
By week 204, a total of 64.4% of patients achieved an IGA 0 or 1, 80.8% achieved a reduction in IGA of ≥2 points from the parent study baseline, and 94.9, 90.9, and 75.8% achieved EASI-50, EASI-75, and EASI-90, respectively (Table 5). Mean EASI improved from week 52 (3.15) to week 204 (2.46), as did mean weekly average Pruritus NRS scores (2.37 at week 52 to 2.10 at week 204). The proportion of patients achieving a ≥3-point improvement in weekly average Pruritus NRS score from parent study baseline at week 204 (78.7%) was similar to that at week 52 (78.2%), whereas the proportion of patients achieving a ≥4-point improvement in weekly average Pruritus NRS score from parent study baseline was greater at week 204 (70.8%) than at week 52 (66.9%).
3.4 Efficacy Following a Switch from Dupilumab 300 mg weekly to 300 mg Every 2 Weeks in the Subgroup of Patients who Switched Doses
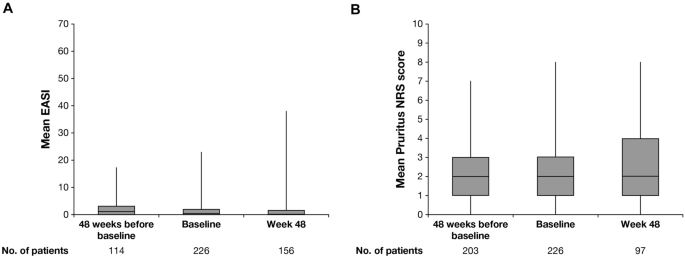
Relative to q2w dosing baseline and 48 weeks prior to dose transition, measures of AD signs and symptoms were stable 48 weeks post-switch among patients who transitioned from qw to q2w dosing (N = 226; Table 5, Fig. 5). The mean EASI at q2w dosing baseline (1.92) was similar to that 48 weeks after the switch (1.93; Table 5, Fig. 5). A similar trend was observed for weekly average Pruritus NRS score (q2w dosing baseline mean score = 2.15, score 48 weeks post-switch = 2.24). Most patients (97.8%) who transitioned from qw to q2w had an NRS score ≤4 (low burden of itch) or EASI ≤7 (mild) at the time of the switch, and more than 80% maintained this stringent response on q2w for 6 months continuously (Fig. 6), indicating well-controlled disease without any exacerbation related to the changed dosing frequency to the approved posology.
Mean A EASI and B Pruritus NRS score of patients switched from dupilumab 300 weekly to 300 every 2 weeks. Baseline of every 2 weeks dosing is defined as last assessment on weekly dose. Vertical bars represent the minimum and maximum values. EASI Eczema Area and Severity Index, NRS Numerical Rating Scale
Kaplan–Meier curve of continuous maintenance of NRS score ≤4 or EASI ≤7 following dupilumab dose switch from 300 mg weekly to 300 mg q2w. Patients maintained response (treated >24 weeks or not) as censored at last assessment within 24 weeks of dose switch. EASI Eczema Area and Severity Index, NRS Numerical Rating Scale, q2w every 2 weeks
4 Discussion
In this study, dupilumab showed an acceptable safety profile and sustained efficacy in adults with moderate-to-severe AD for up to 4 years. Safety data reported here are consistent with data from previous controlled studies of dupilumab up to 52 weeks [10, 12, 19], and common AEs (nasopharyngitis, AD, upper respiratory tract infection, conjunctivitis, headache, oral herpes, and injection-site reactions) are consistent with the known safety profile of dupilumab. These AEs are also similar to those reported in a recent analysis of real-world dupilumab safety data, in which conjunctivitis, blepharitis, keratitis, herpes simplex, and headache were the most commonly reported AEs [27].
Exposure-adjusted incidence rates of TEAEs declined over time, with lower rates in this 4-year analysis (256.86 nE/100 PY) compared with the 3-year analysis of this trial (270.1 nE/100 PY) and an earlier 52-week controlled trial (504.5 nE/100 PY) [10, 17]. Treatment discontinuation because of AEs or lack of efficacy was low (4.3 and 2.2% incidence, respectively), and few patients required rescue treatment (1.8% incidence), indicating well-controlled disease. Compliance with study treatment was high (98.1%).
Clinical trials of dupilumab for the treatment of AD report a greater incidence of ocular surface disease, including conjunctivitis, among patients receiving dupilumab compared with placebo [28, 29]. In this analysis, the exposure-adjusted incidence rate of conjunctivitis was 15.66 nE/PY, which was lower than that reported at 52 weeks in a placebo-controlled trial (30.6 nE/100 PY) and comparable with that reported in the 3-year analysis of this trial (16.14 nE/100 PY) [10, 17]. Most cases of conjunctivitis were mild or moderate in severity, and treatment discontinuation because of conjunctivitis was rare (0.5% of patients). Conjunctivitis incidence also decreased throughout the course of treatment in this study, with the majority of cases (61%) occurring during the first year of dupilumab treatment, and most conjunctivitis events recovered/resolved during the treatment period. These data show that the exposure-adjusted incidence rate of conjunctivitis declined over time. Similar to conjunctivitis, the incidence of injection-site reactions also decreased over time, with a lower incidence in this 4-year analysis (15.11 nE/100 PY) than in the 3-year analysis (16.70 nE/100 PY) and the 52-week controlled trial (78.02 nE/100 PY) [10, 17]. The same was true for skin infections, which decreased from 9.75 nE/100 PY in the 52-week trial to 5.69 nE/100 PY in the 3-year analysis and 5.57 nE/100 PY in the 4-year analysis. These improvements may possibly be due to more efficient control of AD with continued dupilumab treatment.
In this study, dupilumab demonstrated rapid improvements in AD signs and symptoms in the first several weeks of treatment, following by sustained efficacy up to 204 weeks. Sensitivity analyses of EASI and weekly average Pruritus NRS score were consistent with results from analyses on all observed patients, suggesting that patient withdrawal did not bias the results.
To align the OLE study dose regimen with the dose regimen approved by regulatory agencies, patients transitioned from dupilumab 300 mg qw to q2w following Protocol Amendment 9. This subgroup of patients represents the longest treated cohort of patients in the OLE, with a mean total duration of dupilumab exposure of over 4.5 years. Previous controlled studies of dupilumab have demonstrated no differences in safety or efficacy between qw and q2w dose regimens, suggesting that dupilumab 300 mg q2w would achieve results similar to those with 300 mg qw [10, 12, 19]. Indeed, in this study, dupilumab showed sustained efficacy following dose regimen transition from 300 mg qw to 300 mg q2w, with stable signs and symptoms 48 weeks post-transition. Continuous maintenance of disease control, defined as EASI ≤7 or NRS score ≤4 for 24 weeks without exacerbation, was observed in more than 80% of patients who achieved this response at the time of dosage switch.
Strengths of this study include the duration of treatment and overall sample size. Limitations include the open-label design, the absence of a placebo arm, treatment interruptions because of protocol amendments, the smaller subset of patients who received q2w dosing, the smaller subset of patients who transitioned from qw to q2w dosing, and the smaller sample size at later timepoints, which was primarily because of study termination by the sponsor following regulatory approval of dupilumab in the enrollment country. Furthermore, the 300 mg qw dosing regimen is not an approved regimen. Finally, all data reported here correspond to the overall patient population rather than the continuously treated population, which had a smaller sample size at later timepoints.
5 Conclusions
Safety and efficacy results of dupilumab treatment up to 4 years supported dupilumab as a continuous long-term treatment for adults with moderate-to-severe AD. Furthermore, dupilumab efficacy was sustained following dose regimen transition from 300 mg qw to 300 mg q2w.
Change history
06 August 2022
A peer-reviewed video abstract was retrospectively added to this publication.
References
Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
Boguniewicz M, Alexis AF, Beck LA, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5:1519–31.
Schmitt J, Schäkel K, Fölster-Holst R, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. 2010;162:661–8.
Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–78.
Gerbens LAA, Hamann SAS, Brouwer MWD, Roekevisch E, Leeflang MMG, Spuls PI. Methotrexate and azathioprine for severe atopic dermatitis: a 5-year follow-up study of a randomized controlled trial. Br J Dermatol. 2018;178:1288–96.
Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA. 2014;111:5147–52.
Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA. 2014;111:5153–8.
US Food and Drug Administration. DUPIXENT® (dupilumab). Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s014lbl.pdf. Accessed 2 Dec 2021.
European Medicines Agency. DUPIXENT® (dupilumab). Summary of product characteristics. https://ec.europa.eu/health/documents/community-register/2019/20190801145601/anx_145601_en.pdf. Accessed 2 Dec 2021.
Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–303.
Silverberg JI, Simpson EL, Ardeleanu M, et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: a pooled analysis of data from two phase III trials. Br J Dermatol. 2019;181:80–7.
Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–48.
Thaçi D, Simpson EL, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019;94:266–75.
Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56.
Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282–93.
Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377–88.
Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21:567–77.
Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52.
de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178:1083–101.
Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9.
Blauvelt A, Simpson EL, Tyring SK, et al. Dupilumab does not affect correlates of vaccine-induced immunity: a randomized, placebo-controlled trial in adults with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2019;80:158–67.
Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label and subsequent phase III open-label extension. Br J Dermatol. 2020;182:85–96.
Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug–drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146–54.
Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155–72.
Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134:1293–300.
Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:131–43.
Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: a systematic review and meta-analysis. J Am Acad Dermatol. 2021;84:139–47.
Fachler T, Shreberk-Hassidim R, Molho-Pessach V. Dupilumab-induced ocular surface disease: a systematic review. J Am Acad Dermatol. 2021;86:486–7.
Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–73.
Acknowledgements
The authors thank the patients and investigators who participated in the study; Haixin Zhang of Regeneron Pharmaceuticals, Inc., for statistical analyses; and Linda Williams of Regeneron Pharmaceuticals, Inc., and Abby Gan and Tracy Chew of Sanofi, for their contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov identifier: NCT01949311. The study sponsors participated in the study design; collection, analysis, and interpretation of the data; writing of the report; and the decision to submit the article for publication. Medical writing and editorial assistance were provided by Carolyn Ellenberger, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline.
Conflicts of interest
Lisa A. Beck has acted as investigator and/or consultant for AbbVie, Allakos, Arena Pharmaceuticals, AstraZeneca, BenevolentAI, DermTech, Galderma, Inc., Incyte, Janssen, Kiniksa Pharmaceuticals, LEO Pharma, Lilly, Novartis, Numab Therapeutics, Pfizer, Principia Biopharma, RAPT Therapeutics, Regeneron Pharmaceuticals, Ribon Therapeutics, Sanofi, sanofi-aventis, Stealth BioTherapeutics, and Union Therapeutics; and owns stock in 3M, Gilead, Medtronics, and Moderna. Mette Deleuran has received research support, honoraria for lecturing, and/or consulting/advisory board agreements from AbbVie, Arena Pharmaceuticals, Aslan Pharmaceuticals, Eli Lilly, Incyte, La Roche-Posay, LEO Pharma, MSD, Numab Therapeutics, Pierre Fabre, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi. Robert Bissonnette has acted as a consultant for and/or has received grants/research support from AbbVie, Arcutis Biotherapeutics, Arena Pharmaceuticals, Aristea Therapeutics, Asana BioSciences, Bellus Health, Bluefin Biomedicine, Boehringer Ingelheim, Cara Therapeutics, Dermavant, Eli Lilly, EMD Serono, Evidera, Galderma, GSK, Inmagene Biopharmaceuticals, Incyte, Kiniksa Pharmaceuticals, Kyowa Kirin, LEO Pharma, Novan, Pfizer, Ralexar Therapeutics, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Respivant Sciences, Sanofi, Siena Biopharmaceuticals, Target RWE, and Vyne Therapeutics and is a shareholder with Innovaderm Research. Marjolein de Bruin-Weller has acted as a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Arena Pharmaceuticals, Aslan Pharmaceuticals, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc. Ryszard Galus has acted as principal investigator for Boehringer Ingelheim, Dermira, Galderma, Glenmark, Incyte, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi. Takeshi Nakahara has received speaker fees from Maruho and Sanofi. Seong Jun Seo has acted as speaker, investigator, and consultant for AbbVie, Eli Lilly, LEO Pharma, Sanofi. Faisal Khokhar, Jing Xiao, Noah Levit, and Arsalan Shabbir are employees and shareholders or Regeneron Pharmaceuticals, Inc. Jignesh Vakil, Ainara Rodriguez Marco, John O’Malley are employees of and may hold stock and/or stock options in Sanofi.
Ethics approval
This study was conducted in accordance with the ethical standards of the responsible committees and the Declaration of Helsinki and with the International Council for Harmonisation guidelines for good clinical practice. The trial was overseen by an independent data and safety monitoring board. The protocol was reviewed and approved by institutional review boards/ethics committees at all centers.
Consent to participate
Written informed consent was obtained from all patients or their proxies.
Consent for publication
Not applicable.
Availability of data and material
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g., US FDA, European Medicines Agency, Pharmaceuticals and Medical Devices Agency), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Code availability
Not applicable.
Author contributions
LAB, MD, RB, MdeBW, RG, TN, and SJS acquired data. JX conducted the statistical analyses on the data. All authors interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Beck, L.A., Deleuran, M., Bissonnette, R. et al. Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am J Clin Dermatol 23, 393–408 (2022). https://doi.org/10.1007/s40257-022-00685-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-022-00685-0