Abstract
Introduction
Despite the effect on blood lipid levels, the clinical benefits of intensive versus standard pharmacological lipid-lowering therapy remain unclear. Previous reviews have presented relative effects on the risk of clinical outcomes, but not the absolute risk reductions (ARRs) and the time gained to a clinical outcome, also called outcome postponement (OP). The aim of this study was to estimate the effect of intensive versus standard lipid-lowering therapy in terms of ARR and OP of major adverse cardiovascular events (MACE), myocardial infarction (MI), stroke, and all-cause mortality.
Methods
We searched PubMed, Embase, and prior reviews to identify trials comparing intensive versus standard lipid-lowering therapy for the risk of cardiovascular disease. We extracted the number of patients with MACE, MI, stroke, and all-cause mortality. Risk of bias was assessed according to the five domains of the Cochrane Risk of Bias Tool 2.0. We calculated ARRs and OPs to assess the clinical benefits of intensive versus standard therapy for each outcome. We conducted meta-analyses standardizing the results to 2 and 5 years of follow-up.
Results
We identified 11 double-blind, randomized, controlled trials (n = 101,357). The follow-up period ranged from 1.5 to 7.0 years, with an average follow-up duration of 3.7 years. Risk of bias was generally high. During an estimated 2 years of intensive versus standard lipid-lowering therapy, 1.1% (95% confidence interval [CI] 0.7–1.5) fewer patients had MACE and the OP of MACE was 4.1 days (95% CI 2.6–5.6). The effects were 0.7% (95% CI 0.4–0.8) and 2.5 days (95% CI 1.6–3.3) for MI, 0.2% (95% CI 0.1–0.3) and 0.9 days (95% CI 0.5–1.2) for stroke, and 0.2% (95% CI − 0.1 to 0.4) and 0.6 days (95% CI − 0.4 to 1.5) for all-cause death. During on average 5 years of intensive versus standard lipid-lowering therapy, 2.4% (95% CI 1.5–3.3) fewer patients had MACE and the time gained to MACE was 23.5 days (95% CI 14.9–32.0). The effects were 1.5% (95% CI 1.0–2.1) and 14.6 days (95% CI 9.3–20.0) for MI, 0.6% (95% CI 0.4–0.8) and 5.3 days (95% CI 3.3–7.4) for stroke, and 0.4% (95% CI −0.2 to 0.1) and 3.6 days (95% CI − 2.1 to 9.2) for all-cause death.
Conclusion
Intensive lipid-lowering therapy during 2 or 5 years did not lead to fewer deaths or lifetime gained, and the effects on MI and stroke were negligible. The largest effect was that MACE did not occur in two of 100 patients and was postponed 3–4 weeks after 5 years of intensive treatment. Given the small effect, patients should receive this information as part of shared decision making.
Plain Language Summary
Cholesterol-lowering therapy is effective in reducing the risk of heart disease and stroke. Over time, low-density lipoprotein (LDL) cholesterol targets have been progressively lowered to further minimize this risk. This study aimed to compare the effectiveness of intensive versus standard cholesterol-lowering medications for heart disease and stroke benefits. Unlike previous research, this study focused on the absolute difference in risk and the average delay of heart attacks and strokes associated with these treatments. This study analysed data from 11 clinical trials involving over 100,000 participants. It found that intensive cholesterol-lowering medications for 2 or 5 years had a very small impact on heart attacks and strokes and did not lead to fewer deaths or longer lives. The biggest benefit seen was that overall heart disease and strokes did not happen in two out of every 100 patients, and it was delayed by about 3–4 weeks after 5 years of intense treatment. Given the modest effect found in this study, patients should think carefully about this information when making treatment decisions with their doctors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We found that intensive cholesterol-lowering medications for 2 or 5 years had minimal effect on reducing the incidence of heart attacks or strokes. |
The largest effect was that overall heart-related diseases and strokes did not occur in two of 100 patients and their onset was delayed by 3–4 weeks after 5 years of intensive treatment. |
Given this modest effect, patients should consider this information when making treatment decisions with their doctors. |
1 Introduction
The clinical benefit of statin therapy for the reduction of cardiovascular disease has been well established. In the early 2000s, a low-density lipoprotein cholesterol (LDL-C) target of < 100 mg/dL (2.6 mmol/L) was introduced for patients with a high risk of cardiovascular disease in many countries [1]. Subsequently, intensive compared to standard lipid-lowering therapy was found to further reduce the risk of cardiovascular disease. In response to these results, the LDL-C targets were recently set at 70 mg/dL (1.8 mmol/L) for high-risk patients and at 55 mg/dL (1.4 mmol/L) for very high-risk patients. As a result, many patients need more intensive lipid-lowering therapy to reach their LDL-C targets, as nearly half of them fail to do so with standard statin treatment [2].
Intensive lipid-lowering therapy can be achieved with high-dose statins or with ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors added to standard statin therapy. Systematic reviews have reported a 16–19% relative risk (RR) reduction for major adverse cardiovascular events (MACE) [3, 4]. Compared to placebo or less intensive treatment, potent lipid-lowering therapy also reduces the risk of all-cause mortality (RR 0.92; 95% confidence interval [CI] 0.89–0.96), but not the risk of cardiovascular mortality (RR 1.02; 95% CI 0.94–1.09) [5].
Despite reductions in the risk of cardiovascular disease, the clinical relevance of intensive lipid-lowering therapy remains uncertain. First, systematic reviews investigating intensive lipid-lowering therapy may have overestimated the treatment effects of intensive lipid-lowering therapy. These reviews often included trials in which the control group did not receive any form of lipid-lowering therapy. Also, unblinded trials were included. Both approaches could lead to an overestimation of effects on MACE, and so an overestimation of clinical benefit [6].
Second, systematic reviews focussed on the composite outcome MACE [3, 4]. This outcome includes a variety of cardiovascular diseases, revascularization procedures, and hospitalizations. The heterogeneity in this endpoint among trials complicates the interpretation of the results [7]. Also, patients may attribute varying importance to individual MACE components, such as myocardial infarction (MI) or stroke [8, 9]. Furthermore, in preventive medicine, all-cause mortality is an unambiguous and important clinical outcome. Lipid-lowering therapy was once introduced to prevent potentially fatal disease [10]. Also, mortality as an outcome is robust against measurement biases or patient drop-out.
Third, reviews tend to report only RRs or odds ratios without the corresponding absolute effects [3, 4]. Relative effect measures may not fully convey the clinical relevance of a treatment effect, particularly when the baseline risk of an event is low. In such cases, even a minor reduction in the absolute number of events can yield substantial reductions in the RR [11,12,13]. To gain a comprehensive understanding of treatment benefits, both absolute and relative effect measures should be reported, as recommended by Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) guidelines [14].
An absolute risk reduction (ARR) shows how many patients of those treated benefit from the treatment in terms of not experiencing the event of interest during a certain period of treatment. Alternatively, the treatment benefit can be expressed as the amount of additional event-free time gained. Outcome postponement (OP) is an absolute effect measure that can serve this purpose. It represents the average additional event-free time in a group of treated patients. Like ARR, OP offers a more intuitive way to comprehend the benefits of an intervention than RRs [15,16,17].
Several systematic reviews of trials comparing statin therapy to placebo have reported OPs for cardiovascular disease and mortality [18,19,20]. OPs were calculated with survival curves or hazard ratios (HRs) as presented in the included trials. OP to death was found to vary between − 9.5 days and 27.2 days in 11 trials with a follow-up period of 2.0–6.1 years [18]. The pooled OP was 12.6 days (95% CI 7.1–18.0) when the results of 16 trials were included [19]. Another review calculated pooled OPs that were standardized for a follow-up of 5 years. The 5-year OP was 18.0 days (95% CI 12.1–24.1) for MI and 6.1 days (95% CI 2.86–9.39) for stroke [20].
Reporting ARR and OP in addition to RR, respectively, HR, may enhance the understanding of treatment effects and clinical benefits. No systematic review using this approach for intensive versus standard lipid-lowering has been published before. Therefore, the aim of this review was to estimate the OPs and ARRs for MACE, MI, stroke, and all-cause mortality in trials comparing intensive versus standard lipid-lowering therapy.
2 Methods
We conducted a systematic review and meta-analysis of blinded, randomized, controlled trials comparing intensive versus standard lipid-lowering therapy among adult patients with hypercholesterolaemia and an increased risk of cardiovascular disease.
2.1 Search and Selection
One independent reviewer (FB) conducted the search and selection of trials. Two reviewers independently checked the full texts of potentially eligible trials (FB and HJL). We used PubMed and Embase to identify randomized trials comparing intensive versus standard pharmacological lipid-lowering therapy. The search spanned the year 2000 to August 2023, and we used the terms “statin, ezetimibe, PCSK9 inhibitor” and “intensive or high-dose”. Additionally, we hand-searched the references of three recent systematic reviews about intensive lipid-lowering therapy trials [3,4,5].
When the title and abstract of a hit suggested a potentially eligible trial, we obtained and assessed the full-text publication. We selected randomized, placebo-controlled trials that compared intensive versus standard therapy among adult patients at an increased risk of cardiovascular disease. Intensive lipid-lowering therapy was defined as the use of a high-dose statin, more potent statin, ezetimibe added to statin treatment, or a PCSK9 inhibitor added to statin and/or ezetimibe therapy. Standard lipid-lowering therapy could be a standard dose of a statin, a less potent statin, or a combination of a standard statin and ezetimibe. Trials were included irrespective of sample size, type of population (primary or secondary prevention), or duration of follow-up.
We excluded trials that investigated an intensive lipid-lowering therapy that is rarely used such as bile acid sequestrants or based on a drug without market authorization such as bococizumab. Also, as HRs were necessary to calculate OP, trials not reporting an HR for at least one of the four outcomes were excluded.
2.2 Risk of Bias Assessment
The risk of bias assessment was conducted independently by two reviewers (FB and DL). Following the Cochrane Risk of Bias Tool 2.0, we assessed five domains that could potentially influence the observed treatment effects: the randomization process, deviation from the intended intervention, missing outcome data, outcome measurement, and selective reporting. Unblinded adjudication by a central committee was assessed as part of outcome measurement. We added a sixth domain about the presence of a run-in period. Selecting highly compliant and side-effect-tolerant patients can potentially inflate the estimated treatment effect [21]. A domain was categorized as having a low risk of bias when the study's design, conduct, and results did not suggest the presence of bias; it was categorized as having a high risk if there were indications of bias; and it was categorized as having an unclear risk when information was insufficient with potential concerns about bias. Any disagreements were resolved through consensus meetings. No study protocol was registered.
2.3 Data Extraction
Two independent reviewers (FB and DL, or FB and EH) extracted the data of interest. These were general characteristics including the type of intensive and standard lipid-lowering therapy, number of participants per group, trial duration, mean or median follow-up, and commercial funding.
Next, we extracted the number of participants in each comparison group who had MACE, MI, or stroke or who died. MACE was the primary outcome of most included trials. Stroke may have included ischaemic and haemorrhagic stroke. If only non-fatal MI or non-fatal stroke were reported, we considered the outcome as missing. Also, the HRs (or RRs as a proxy) and their 95% CIs for the outcomes were extracted. In addition, the Kaplan-Meier survival curves for MACE were extracted to enable a mathematical check (see below).
2.4 Statistical Analysis
To estimate the ARR and OP, we applied a previously described methodology [19]. This approach involves a mathematical model of the area between survival curves. The model incorporates event rates in the control group, the reported HR for the intervention compared to the control group, and the time period for which one would like to estimate the effect. It assumes stable event rates (proportional hazards) in both treatment and control arms during the trial. To estimate OPs, we inputted the maximum follow-up of the included trials in the model. To obtain an ARR, we first estimated the survival rate in the control group using an exponential decay model based on the control group's event rate and the maximum follow-up. If the event rate in the control group was not provided, we calculated it using a hierarchy, as previously described [19]. We then calculated the survival rate in the intervention group by multiplying the survival rate in the control group with the reported HR. The ARR was determined by subtracting the survival rate in the intervention group from that in the control group. We determined the CIs by substituting the lower and upper confidence limit of the HR into the model [19]. Like the ARR, the OP and its CIs were calculated using an exponential decay model based on the control group's event rate, maximum follow-up, and reported HR.
The use of the maximum follow-up for the calculation of ARRs and OPs allowed us to calculate OP with a second method and verify the results of the HR-based method. In this approach, Kaplan–Meier survival curves are reconstructed into individual patient data. To do so, we used the WebPlotDigitizer tool, which is a freely available web-based tool [22], and an iterative least squared technique [23, 24]. We calculated the restricted mean survival time (RMST) with 95% CI using the strmst2 command in Stata. This command also provides the difference in RMST between groups, which is equal to OP, along with the 95% CI. We only calculated RMST for MACE as Kaplan-Meier survival curves for other cardiovascular outcomes were mostly not available.
2.5 Meta-Analysis
We performed a meta-analysis to pool the reported HRs, and the calculated ARRs and OPs for the outcomes of interest. We used a random-effect model for all outcomes, regardless of the observed I2. This decision was motivated by the heterogeneous nature of the included studies related to baseline cardiovascular risk, the type of medications used in the intervention and control groups, the duration of follow-up, and the definition of MACE.
As ARRs and OPs strongly depend on trial duration [20], we standardized the trial duration across trials for the most common durations observed in the included studies, namely 2, 3, and 5 years. To do so, we calculated the ARRs and OPs and their 95% CIs per individual trial using a follow-up period of 2, 3, and 5 years, respectively. Subsequently, we pooled these standardized ARRs and OPs. In addition, we conducted sensitivity analyses with trials that had a trial duration that was equal to or longer than 2, 3, or 5 years. This allowed us to estimate the potential impact of extrapolating outcomes for trials with a follow-up shorter than 2, 3, and 5 years, respectively.
Finally, in line with GRADE recommendations for a summary of findings table, we also calculated the number of affected persons per 1000 participants in the control and intervention groups. For the latter, a 95% CI was also provided. We performed the statistical analyses with Stata [25].
3 Results
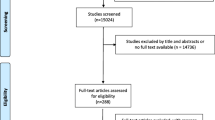
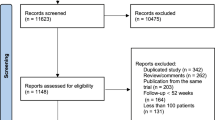
Of 63 potentially eligible trials, we included 11 trials about intensive versus standard lipid-lowering therapy, with 101,357 participants (Fig. 1) [26,27,28,29,30,31,32,33,34]. The excluded trials did not report an HR or RR for one or more of the outcomes of interest (24 trials), used placebo or usual care as a comparator without standard lipid-lowering therapy (16), or were open-label trials (12).
In the 11 included trials, the intervention group received different intensive lipid-lowering treatments: more potent or higher dosage statins in four trials, ezetimibe in one trial, and a PCSK9 inhibitor in six trials (Table 1). Except for one trial (SEARCH), all included studies received funding from pharmaceutical companies. Ten studies were found to have a high risk of bias in at least one domain, mostly related to small baseline differences in favour of the investigated treatment or unblinded central adjudication (see Supplemental Table 1 in the electronic supplementary material). The cardiovascular events of the ORION9-11 trials were only available as a pooled figure.
3.1 Effects per Trial
Table 2 presents the effects of intensive versus standard lipid-lowering therapy on the risk of MACE for each included trial. The trial duration varied between 1.5 and 7.0 years (average 3.7 years), with eight out of 11 trials having a relatively short follow-up of 4 years or less. HRs indicated a 6% reduction (HR 0.94; 95% CI 0.88–1.01) to a 48% reduction (HR 0.52; 95% CI 0.31–0.90). The ARRs ranged from 1.2% (95% CI − 0.2 to 2.5; maximum follow-up 7.0 years) to 3.9% (95% CI 1.2–6.4; maximum follow-up 2.5 years). Average delays in time to MACE varied between 5.2 days (95% CI 1.1–7.4; maximum follow-up 1.7 years) and 29.9 days (95% CI 14.9–42.2; maximum follow-up 6.0 years). In general, OPs tended to increase with longer follow-up periods, as expected, but some trials deviated from this trend. The OPs for MACE were consistent with those obtained from the RMST-method (see Supplemental Table 2 in the electronic supplementary material).
Table 2 also presents the results for the other cardiovascular outcomes and all-cause mortality. For MI, HRs ranged from a 4% reduction (HR 0.96; 95% CI 0.77–1.21 [AZ]; HR 0.96; 95% CI 0.90–1.04 [SEARCH]) to a 27% reduction (HR 0.73; 95% CI 0.65–0.82). ARRs varied between 0.3% (95% CI − 1.4 to 1.6; maximum follow-up 2.0 years) and 1.9% (95% CI 0.7–1.9; maximum follow-up 6.0 years). The smallest gain in OP was 1.0 days (95% CI − 5.4 to 6.0; maximum follow-up of 2.0 years), while the largest was 21.8 days (95% CI 8.4–33.7; maximum follow-up of 7.0 years). Again, OPs tended to increase with longer follow-up periods.
For stroke, HRs ranged from a reduction of 9% (HR 0.91; 95% CI 0.77–1.08) to a 27% reduction (HR 0.73; 95% CI 0.57–0.93). ARRs ranged from −0.1% (95% CI − 0.9 to 0.6; maximum follow-up of 2.5 years) to 0.9% (95% CI 0.1–1.5; maximum follow-up 6.0 years). The smallest average delay in stroke was −0.5 (95% CI − 4.3 to 2.9; maximum follow-up 2.5 years), while the largest was 10.2 days (95% CI 1.6–16.7; maximum follow-up of 6.0 years).
For all-cause mortality, HRs ranged from an increase of 4% (HR 1.04; 95% CI 0.91–1.19) to a 15% reduced risk (HR 0.85; 95% CI 0.73–0.98). ARRs ranged from − 0.2% (95% CI − 0.8 to 0.4; maximum follow-up 3.0 years) to 1.3% (95% CI − 0.1 to 2.5; maximum follow-up 2.0 years). The average delay of death ranged from − 0.9 days (95% CI − 4.3 to 2.0; maximum follow-up of 3.0 years) to 9.8 days (95% CI − 0.7 to 18.3; maximum follow-up of 2.5 years).
3.2 Pooled Effects
Table 3 presents the pooled HRs, and ARRs and OPs standardized to 2 and 5 years of follow-up. Pooled effects for 3 years of follow-up were very similar to those for 2 and 5 years and can be found in Supplemental Table 3 (see the electronic supplementary material). The pooled HR for MACE was 0.87 (95% CI 0.82–0.91; I2 56.2%). When standardized to 2 years of follow-up, the ARR was 1.1% (95% CI 0.7–1.5; I2 61.9%) and the OP was 4.1 days (95% CI 2.6–5.6; I2 62.5%). For 5 years of follow-up, the ARR increased to 2.4% (95% CI 1.5–3.3; I2 59.6%) and the OP to 23.5 days (95% CI 14.9–32.0; I2 61.0%).
The pooled HR for MI was 0.86 (95% CI 0.80–0.93; I2 61.0%). After 2 years of follow-up, the ARR was 0.7% (95% CI 0.4–0.9; I2 39.7%) and the OP was 2.5 days (95% CI 1.6–3.3; I2 39.4%). Standardizing to 5 years of follow-up resulted in an ARR of 1.5% (95% CI 1.0–2.1; I2 41.0%), along with an OP of 14.6 days (95% CI 9.3–20.0; I2 40.2%). The pooled HR for stroke was 0.83 (95% CI 0.76–0.90; I2 0.0%). When standardized to 2 years of follow-up, the ARR was 0.2% (95% CI 0.1–0.3; I2 0%) and the OP was 0.9 days (95% CI 0.5–1.2; I2 0%). For 5 years of follow-up, the ARR increased to 0.6% (95% CI 0.4–0.8; I2 0%), along with a delay of 5.3 days (95% CI 3.3–7.4; I2 0%).
No statistically significant effects were observed for all-cause mortality. The pooled HR was 0.97 (95% CI 0.92–1.02; I2 0.0%). After 2 years of follow-up, the ARR was 0.2% (95% CI − 0.1 to 0.4; I2 49.7%) and the OP was 0.6 days (95% CI − 0.4 to 1.5; I2 49.8%). For 5 years of follow-up, the ARR was 0.4% (95% CI − 0.2 to 1.0; I2 48.9%) and the delay was 3.6 days (95% CI − 2.1 to 9.2; I2 49.4%).
Subgroup analysis with just trials that exceeded the standardized duration of follow-up did not yield substantially different effect sizes across outcomes at 2 and 3 years of follow-up. However, for a duration of follow-up > 5 years, the ARRs and OPs for MACE and MI were notably smaller when only trials with a longer duration were included (Supplemental Tables 4–6). For MACE, the ARR was 1.5% (95% CI 0.7–2.2) and the OP was 14.1 days (95% CI 7.1–21.1). For MI, the ARR was 1.1% (95% CI 0.6–1.7) and the OP was 10.8 (95% CI 5.9–15.8).
4 Discussion
In this study, we calculated absolute reductions in the risk of cardiovascular outcomes and OP in days for 11 double-blind trials comparing intensive versus standard lipid-lowering therapy. The largest ARR for MACE was 3.9% in a trial with a duration of 2.5 years, and the largest OP was 29.9 days in a trial of 6.0 years. When pooling ARRs and OPs for a standardized 5-year follow-up period, the effects were 2.4% and 23.5 days for MACE, 1.5% and 14.6 days for MI, and 0.6% and 5.3 days for stroke. For all-cause mortality, no effect was observed.
4.1 Relative Versus Absolute Treatment Effects
Our study showed that substantial relative reductions in HRs corresponded with small absolute effects of intensive versus standard lipid-lowering therapy. Similar results for cardiovascular outcomes were reported in a systematic review that compared pooled HRs to OPs for trials assessing statin therapy versus placebo [20] and in two studies that calculated OPs for MACE in individual trials about intensive versus standard lipid-lowering therapy (IMPROVE-IT trial, FOURIER, and ODYSSEY OUTCOMES) [23, 35]. This apparent discrepancy can be attributed to the low baseline incidence of cardiovascular events in the included trials. Trials with higher baseline event rates may yield more substantial event delays. This was for instance observed in the CONSENSUS trial, which compared enalapril to placebo in patients with congestive heart failure New York Heart Association (NYHA) class IV. For the outcome death, the ARR was 16.7% and the corresponding OP 5 months during a mean follow-up period of 6 months [35, 36].
4.2 Limited Clinical Benefit
We found that the absolute reduction in the risk of MACE was 2.4% per 5 years and the average delay 24 days. This implies that the clinical benefit of intensive versus standard lipid-lowering therapy is rather limited. In 12 studies about patient preferences for preventive cardiovascular medication, 28–58% of patients would not consider taking medication if the 5-year ARR was less than 3% [37]. The communication of event postponement may reduce the support of policy makers and healthcare insurers [38]. Also, statin users redeemed prescriptions less often when their general practitioner had communicated treatment effectiveness using postponement of death instead of the ARR [39].
The limited clinical benefit of intensive lipid-lowering therapy questions current recommendations in guidelines. These recommendations focus on attaining LDL-C targets of 70 mg/dL (1.8 mmol/L) for high-risk patients and 55 mg/dL (1.4 mmol/L) for very high-risk patients. Both targets are lower than what is considered biologically normal (77 mg/dL or 2.0 mmol/L). To reach these LDL-C targets, nearly half of patients may require intensive lipid-lowering therapy [4]. Considering the limited clinical benefits of such therapy, and variation in patient preferences, the absolute effects need to be discussed with the patients as part of shared decision making.
4.3 Strengths and Limitations
This study is the first to report pooled HRs with the corresponding pooled ARRs and OPs for trials comparing intensive versus standard lipid-lowering therapy for the risk of cardiovascular disease. Previous studies have used a similar approach for trials comparing statins to placebo [18, 20]. Our study expands on these results, providing a comprehensive insight in the clinical benefits of intensive lipid-lowering therapy to reduce the risk of cardiovascular disease. Another strength of our study is that we omitted the results of open-label trials. Lack of blinding in clinical trials may bias the estimated effects on outcomes that depend on (subjective) clinical diagnostic decisions and interpretations [6]. Examples are hospital admissions and percutaneous coronary interventions, which are often components of MACE.
Our study has a limitation concerning the applicability of our results to long-term use. Most of the included trials had a follow-up duration of less than 4 years, while intensive lipid-lowering therapy is commonly prescribed for a much longer time in clinical practice. Another limitation is that the results for OP apply to all included patients rather than specifically to individuals affected by a clinical event. It is also worth noting that in patients who experience an event, the magnitude of the event delay can be much larger than the OP [40]. However, identifying these individuals when initiating therapy is still impossible.
5 Conclusion
There were substantial RR reductions for cardiovascular disease in trials comparing intensive versus standard lipid-lowering therapy, but the corresponding absolute reduction in risk and time gained to an outcome were small. This indicates a limited treatment benefit. Reporting ARRs and OPs in clinical trials and systematic reviews may provide a more comprehensive understanding of the health benefits of treatments. If these benefits are limited, patients should receive this information as part of shared decision making.
References
Grundy SM, Cleeman JI, BaireyMerz CN, Brewer HB, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39.
Akyea RK, Kai J, Qureshi N, Iyen B, Weng SF. Sub-optimal cholesterol response to initiation of statins and future risk of cardiovascular disease. Heart. 2019;105:975–81.
Wang N, Fulcher J, Abeysuriya N, Park L, Kumar S, Di Tanna GL, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020;8:36–49.
Toyota T, Morimoto T, Yamashita Y, Shiomi H, Kato T, Makiyama T, et al. More-versus less-intensive lipid-lowering therapy: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2019;12.
Ennezat PV, Guerbaai RA, Maréchaux S, Le Jemtel TH, François P. Extent of low-density lipoprotein cholesterol reduction and all-cause and cardiovascular mortality benefit: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2023;81:35–44.
Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk-of-bias assessments and results of randomized trials in cochrane reviews: the ROBES meta-epidemiologic study. Am J Epidemiol. 2018;187:1113–22.
Ferreira-González I, Permanyer-Miralda G, Busse JW, Bryant DM, Montori VM, Alonso-Coello P, et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60:651–7.
Ahmad Y, Nijjer S, Cook CM, El-Harasis M, Graby J, Petraco R, et al. A new method of applying randomised control study data to the individual patient: a novel quantitative patient-centred approach to interpreting composite end points. Int J Cardiol. 2015;195:216–24.
Goldberg R, Gore JM, Barton B, Gurwitz J. Individual and composite study endpoints: separating the wheat from the chaff. Am J Med. 2014;127:379–84.
Ozdemir S, Baid D, Verghese NR, Lam AY, Lee PC, Lim AY, et al. Patient preferences for medications in managing type 2 diabetes mellitus: a discrete choice experiment. Value Heal. 2020;23:842–50.
Doi SA, Furuya-Kanamori L, Xu C, Lin L, Chivese T, Thalib L. Controversy and debate: questionable utility of the relative risk in clinical research: paper 1: a call for change to practice. J Clin Epidemiol. 2022;142:271–9.
Leung WC. Balancing statistical and clinical significance in evaluating treatment effects. Postgrad Med J. 2001;77:201–4.
Noordzij M, Van Diepen M, Caskey FC, Jager KJ. Relative risk versus absolute risk: one cannot be interpreted without the other. Nephrol Dial Transpl. 2017;32:ii13–8.
Schünemann H, Brozek J, Oxman A E. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2. 2009.
Morris J, Hammitt JK. Using life expectancy to communicate benefits of health care programs in contingent valuation studies. Med Decis Mak. 2001;21:468–78.
Christensen PM, Brosen K, Brixen K, Andersen M, Kristiansen IS. A randomized trial of laypersons’ perception of the benefit of osteoporosis therapy: number needed to treat versus postponement of hip fracture. Clin Ther. 2003;25:2575–85.
Halvorsen PA, Selmer R, Kristiansen IS. Different ways to describe the benefits of risk-reducing treatments: a randomized trial. Ann Intern Med. 2007;146:848–56.
Kristensen ML, Christensen PM, Hallas J. The effect of statins on average survival in randomised trials, an analysis of end point postponement. BMJ Open. 2015;5:e007118.
Hansen MR, Hróbjartsson A, Pottegård A, Damkier P, Larsen KS, Madsen KG, et al. Postponement of death by statin use: a systematic review and meta-analysis of randomized clinical trials. J Gen Intern Med. 2019;34:1607–14.
Hansen MR, Hróbjartsson A, Pottegård A, Damkier P, Madsen KG, Pareek M, et al. Postponement of cardiovascular outcomes by statin use: a systematic review and meta-analysis of randomized clinical trials. Basic Clin Pharmacol Toxicol. 2021;128:286–96.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Version 5. The Cochrane Collaboration, 2011; 2019.
WebPlotDigitizer—extract data from plots, images, and maps. http://arohatgi.info/WebPlotDigitizer/.
Messori A, Bartoli L, Chiumente M, Mengato D, Trippoli S. The restricted mean survival time as a tool for ranking comparative outcomes in a narrative review that evaluates a network of randomized trials: an example based on PCSK9 inhibitors. Am J Cardiovasc Drugs. 2021;21:349–54.
Messori A, Trippoli S, Vaiani M, Cattel F. Survival meta-analysis of individual patient data and survival meta-analysis of published (aggregate) data: is there an intermediate approach between these two opposite options? Clin Drug Investig. 2000;20:309–16.
StataCorp. Stata statistical software: release 17. College Station: StataCorp LLC; 2021.
La Rosa JC, Conti CR. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. ACC Cardiosource Rev J. 2006;15:97–9.
Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504.
De Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KAA, White HD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–16.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023;44:129–38.
Webster J, Henderson J, Nixon A, Lackie S, Thompson J, Rhoden W, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–69.
Ennezat PV, Alavi Z, Le Jemtel TH, Hansen MR. Consideration regarding the analysis of randomized controlled trials in the era of evidence-based medicine. J Cardiovasc Pharmacol. 2022;79:605–19.
Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol. 1988;62:60A-66A.
Albarqouni L, Doust J, Glasziou P. Patient preferences for cardiovascular preventive medication: a systematic review. Heart. 2017;103:1578–86.
Jaspers NEM, Visseren FLJ, Numans ME, Smulders YM, Van Loenen Martinet FA, Van Der Graaf Y, et al. Variation in minimum desired cardiovascular disease-free longevity benefit from statin and antihypertensive medications: a cross-sectional study of patient and primary care physician perspectives. BMJ Open. 2018;8:e021309.
Harmsen CG, Kristiansen IS, Larsen PV, Nexøe J, Støvring H, Gyrd-Hansen D, et al. Communicating risk using absolute risk reduction or prolongation of life formats: cluster-randomisedtrial in general practice. Br J Gen Pract. 2014;64:e199.
Lytsy P, Berglund L, Sundström J. A proposal for an additional clinical trial outcome measure assessing preventive effect as delay of events. Eur J Epidemiol. 2012;27:903–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Folkert H. van Bruggen, Esther C. de Haas, Sytse U. Zuidema, and Hendrika J. Luijendijk have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Authors' contributions
FHvB designed the study, performed the search, extracted the data, assessed the risk of bias, analysed the data, and drafted the manuscript. ECdH helped design the study, helped analyse the data, and provided feedback on the manuscript. SUZ provided feedback on the manuscript on several occasions. HJL helped design the study, performed the search, extracted data, assessed risk of bias, and helped draft the manuscript. HJL is the guarantor of the paper and affirms that the article is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. All authors have approved the final manuscript and consented to its publication.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval
Not applicable.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van Bruggen, F.H., de Haas, E.C., Zuidema, S.U. et al. Time Gained to Cardiovascular Disease by Intensive Lipid-Lowering Therapy: Results of Individual Placebo-Controlled Trials and Pooled Effects. Am J Cardiovasc Drugs (2024). https://doi.org/10.1007/s40256-024-00668-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40256-024-00668-y