Abstract
Purpose
The role of sodium-glucose cotransporter 2 inhibitors (SGLT2i) in managing cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM) is evolving. This meta-analysis seeks to explore the influence of SGLT2i on the recurrence of atrial fibrillation (AF) following catheter ablation (CA) in individuals with T2DM qualitatively and quantitatively.
Methods
A comprehensive literature search was conducted in electronic databases. Studies meeting predefined criteria were included. Individual patient data (IPD) were used from reconstructed time-to-event data to estimate hazard ratios (HRs) and 95% confidence intervals for AF recurrence. IPD meta-analysis was followed by a direct meta-analysis to assess the risk of AF recurrence.
Results
A total of five studies [one randomized controlled trial (RCT) and four cohort studies] were included in this study, and five studies were included in the qualitative analysis, while four studies comprising 1043 patients with T2DM were included in the quantitative analysis. The pooled Kaplan–Meier curve based on reconstructed data showed a significantly lower risk of AF recurrence in the SGLT2i group compared with all antidiabetic drugs (log-rank P = 0.00011) and dipeptidyl-peptidase IV inhibitors (DPP4i) (log-rank P = 0.01). Cox regression analysis showed consistent results. Direct meta-analysis showed that SGLT2i, compared with all antidiabetic medications (HR 0.57, 95% CI [0.44, 0.73], I2) and DPP4i (HR 0.41, 95% CI [0.24, 0.70], I2), was associated with a lower risk of AF recurrence.
Conclusions
SGLT2i are associated with a reduced risk of AF recurrence after CA in patients with T2DM. These results suggest that SGLT2i is promising in improving clinical outcomes for this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The findings of our research suggest that administering sodium-glucose cotransporter 2 inhibitors is linked to a reduced likelihood of atrial fibrillation recurrence following catheter ablation in patients with diabetes. |
1 Introduction
Atrial fibrillation (AF) is the most commonly encountered arrhythmia in clinical practice, and it is well established that there is a strong link between AF and type 2 diabetes mellitus (T2DM). Extensive research has consistently shown that individuals with T2DM are at a 34% higher risk of developing AF compared with those without diabetes [1]. Individuals with longstanding T2DM who have had difficulty maintaining good glycemic control tend to face a greater risk of AF [2]. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have attracted considerable attention owing to their therapeutic efficacy in the management of T2DM, heart failure, and myocardial infarction [3, 4]. Notably, SGLT2i have been found to prevent AF occurrence in T2DM, as evidenced by multiple comprehensive meta-analyses [5]. A meta-analysis involving 16 randomized-controlled trials (RCTs) showed that SGLT2i can reduce the risk of developing new-onset AF in patients with T2DM, regardless of age, body weight, HbA1c, and systolic blood pressure at baseline [6].
Catheter ablation (CA) is a commonly employed treatment strategy for patients with AF with significant symptoms, recurrent AF, or drug contraindications. Catheter ablation is recognized as a safe and effective treatment for AF that has greatly improved patient quality of life [7]. Prior studies suggest that T2DM independently predicts AF recurrence after CA [8]. The role of diabetes medications in AF recurrence after CA is unclear. Observational studies have suggested that certain antidiabetic medications, such as metformin and pioglitazone, are associated with a reduced risk of AF recurrence following CA with a reduced risk of AF recurrence following CA [9, 10]. Conversely, another study found no association between metformin, sulfonylurea, or insulin use and AF recurrence after CA [11].
There are numerous effects of SGLT2i that confer benefits to patients with cardiovascular pathologies. These include improved hemodynamic control and myocardial energy supply and the regulation of autonomic nervous system activity [12]. As such, it would be interesting to investigate the impact of SGLT2i on the recurrence of AF following catheter ablation. Recently, some studies have explored the role of SGLT2i in suppressing AF recurrence after CA. These studies have demonstrated promising results, as SGLT2i were associated with a statistically significant delay in AF recurrence compared with other antidiabetic medications [13,14,15,16]. However, these studies are limited by their small sample sizes. Therefore, we aim to address this limitation by conducting an individual patient meta-analysis using the reconstruction of Kaplan–Meier curves. This method has the ability to overcome limitations in secondary analyses of survival data, such as the need for alternative measures when the proportional hazard ratio assumption does not hold and the potential for bias in meta-analyses conducted using aggregated summary statistics [3]. The primary aim of our study is to examine the efficacy of SGLT2i on AF recurrence after CA in patients with T2DM [17].
2 Methodology
2.1 Literature Search and Data Collection
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. We comprehensively searched four electronic databases: PubMed, Web of Science, Scopus, and CENTRAL. We also conducted a non-systematic search in Google Scholar. The search strategy employed the following keywords: ((SGLT2i) OR (sodium-glucose cotransporter 2 inhibitors) OR (gliflozins) OR (dapagliflozin) OR (canagliflozin) OR (empagliflozin) OR (tofogliflozin)) AND ((atrial fibrillation) OR (AF) OR (atrial flutter) OR (AFib)) AND ((catheter ablation) OR (CA) OR (radiofrequency ablation) OR (RFA) OR (pulmonary vein isolation) OR (PVI)) AND ((type 2 diabetes mellitus) OR (T2DM)). Duplicates were removed using Endnote software, and all retrieved articles underwent a two-step screening process involving title/abstract and full-text assessment. Only articles that met the predefined eligibility criteria were included in the study.
2.2 Eligibility Criteria and Study Selection
The screening process was conducted using Rayaan software. Two independent reviewers (Y.S. and A.M.) assessed the articles for eligibility. We included studies that met the following criteria: (1) RCTs or observational studies, (2) patients having diabetes with atrial fibrillations who have undergone catheter ablation, (3) intervention involving SGLT2i after ablation, (4) comparator consisting of any other antidiabetic drug, and (5) study subjects being humans of any age or sex. Studies without available full texts or with only abstracts were excluded.
2.3 Methodological Quality Assessment
For RCTs, we assessed the risk of bias using the Risk of Bias Assessment tool-2 (ROB2) [19]. This tool evaluates multiple domains, including selection bias, blinding of participants and personnel, blinding of outcome assessment, selective reporting, incomplete outcome data, and other sources of bias. The risk of bias judgment was categorized as low, with some concerns, or high. We used the Newcastle Ottawa Scale to assess the methodological quality of observational studies [20]. We assessed the quality of observational studies on the basis of the following domains: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, comparability, outcome assessment, and follow-up. We added a star for each of the following conditions: the exposed cohort well represents the community, the non-exposed cohort is chosen from the same population, proper assessment for the presence of the exposure, absence of the outcome at baseline, no significant baseline difference between the two groups, proper assessment of the outcome including adequate tools so as not to miss asymptomatic cases, adequate duration of follow-up to detect late recurrence, and no significant loss to follow-up.
2.4 Data Extraction
Two independent reviewers (Y.S. and B.E.) performed data extraction. We used an Excel sheet to extract relevant information, including the study design, study population characteristics, risk of bias assessment, and the primary outcome of AF recurrence reported as the hazard ratio, odds ratio, or raw numbers. To extract data from Kaplan–Meier curves for subsequent Kaplan–Meier curve reconstruction, we utilized Engauge Digitizer software for Microsoft Windows.
2.5 Data Synthesis
We used qualitative and quantitative methods in data synthesis. Qualitative data analysis involved description of the studies while quantitative analysis involved data analysis using meta-analysis. Data analysis was conducted using RStudio, version 4.2.2 for Microsoft Windows. We employed various libraries for different analysis steps, including “tidyverse” for data wrangling, “IDPfromKM” for Kaplan–Meier curve reconstruction, “survival”, “K.M. surv”, and “survminer” for running survival analysis using the reconstructed individual patient data (IPD), “ggplot2” for data visualization of the bar charts, and “meta” for performing the meta-analysis. The reconstruction of IPD involved two steps. Firstly, we extracted data coordinates for time and survival probabilities from Kaplan–Meier curves using Engauge Digitizer software. Secondly, we preprocessed the extracted time and survival probabilities to reconstruct IPD in the form of (time, status) pairs, ensuring accuracy through the iterative algorithm introduced by Liu et al. [17].
To evaluate the precision of the reconstructed IPD, we established the following criteria: a root mean square error of ≤ 0.05, a mean absolute error of ≤ 0.02, and a maximum absolute error of ≤ 0.05. Statistical tests, such as the Mann–Whitney test and bootstrap t-test, were utilized to compare the distribution of the read-in data and the newly estimated survival curve, with a higher P value indicating better concordance. Additionally, a visual inspection was performed to verify the consistency between the reconstructed IPD Kaplan–Meier curves and the original curves. Subsequently, data from all included studies were pooled for further analysis.
In the data analysis, a pooled Kaplan–Meier curve was constructed using all patients to assess the efficacy of SGLT2i in AF recurrence. The significance between the two groups was evaluated using the log-rank test. Cox regression analysis was performed for the study by Kishima et al. 2022 that did not report the hazard ratio to enable us to pool hazard ratios from all studies in a direct meta-analysis [15]. Furthermore, the Chi-squared test was employed on the original IPD data to analyze the statistical significance of the differences in recurrence and non-recurrence frequencies. A P value < 0.05 was considered statistically significant.
With regards to the analysis of the efficacy of SGLT2i versus DPP4i as well as SGLT2i versus all antidiabetic medications, we utilized the DPP4i arm in Liu et al. 2022 and Kishima et al. 2022 to analyze the efficacy of SGLT2i against DPP4i. Then, we performed a second analysis of SGLT2i versus all antidiabetic medications. We were not able to make any other comparisons because they were not done in the primary studies included.
Following the survival analysis, a direct meta-analysis with a random-effects model was conducted using hazard ratios reported in the included studies, except for one study [15] that required us to run a Cox regression model on the raw data to obtain its hazard ratio. Heterogeneity was assessed using I-squared and Chi-squared P values, with I-squared values ≥ 50% and Chi-squared P values < 0.1 indicating significant heterogeneity. In the presence of significant heterogeneity, sensitivity analysis using the leave-one-out model was performed to explore the sources of heterogeneity. Finally, trial sequential analysis (TSA) was conducted to evaluate the robustness of the evidence and assess the need for additional trials. TSA methodology addresses type I errors by identifying insufficient information size and highlighting potentially inconclusive results (i.e., falsely positive or negative). Similar to interim analyses in randomized trials, TSA analyzes data in chronological order to determine whether the available evidence definitively supports a pre-specified treatment effect or lack thereof. Additionally, TSA helps distinguish between truly negative results (indicating no treatment effect) and falsely negative findings (due to inadequate power) in the presence of multiple testing. [21]
3 Results
3.1 Search Results and Study Selection
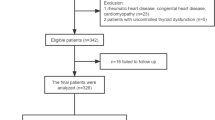
A total of 355 records were identified through the databases. After removing duplicates, 345 records underwent title and abstract screening and 330 records were excluded, leaving 15 articles for full-text screening. After further evaluation, ten articles were excluded, resulting in a total of five eligible studies for qualitative synthesis; four of these were included in the meta-analysis (Fig. 1).
3.2 Characteristics of Included Studies
This systematic review included a total of five studies comprising 5493 patients with T2DM [13,14,15,16]. Of these studies, four studies comprising 1043 patients were eligible to be included in our meta-analysis. The study by Abu-Qaoud et al. [16] was not eligible for quantitative synthesis (meta-analysis) as this study reported a composite outcome that did not include AF recurrence but was included in the qualitative synthesis part of our study. The primary outcome of this study was a composite of cardioversion, new antiarrhythmic drug (AAD) therapy, or re-do AF ablation. SGLT2i were associated with a significantly lower risk of cardioversion, new AAD therapy, and re-do AF ablation (OR 0.68, 95% CI 0.60, 0,77) compared with control.
Regarding the other studies, one study was a RCT [15], three were retrospective cohort studies [13, 14, 16], and one was a prospective cohort study [22]. The studies were conducted in China, Japan, Taiwan, and the USA. The SGLT2i employed in the studies were empagliflozin, canagliflozin, dapagliflozin, and tofogliflozin. In Liu et al. 2023, the analysis was performed by comparing SGLT2i with either dipeptidyl peptidase IV inhibitors (DPP4i) or all other antidiabetic drugs separately, yielding consistent results favoring SGLT2i [14]. Anagliptin, a DPP4i, was the control in Kishima et al. [15], while the control in Luo et al. [13] included various antidiabetic medications other than SGLT2i [13]. Zhao et al. did not mention the type of SGLT2i, and the control included various antidiabetic medications other than SGLT2i [22]. Detailed baseline characteristics of the patients are presented in Table 1.
3.3 Risk of Bias
According to the Newcastle Ottawa Scale for observational studies, three included studies were of good quality, while only one was of poor quality (Table S1). The included RCT was low quality according to the Risk of Bias Assessment tool-2 (ROB2) (Table S2).
3.4 Survival Analysis from Reconstructed KM Curves
3.4.1 SGLT2i versus Antidiabetic Drugs
A detailed summary of our statistical analysis findings is presented in Table 2. In the control group, there were 743 patients with 146 (39.03%) events (AF recurrence), while the SGLT2i group consisted of 300 patients with 34 (23.00%) events. The survival analysis based on reconstructed KM curves revealed a statistically significant difference between the SGLT2i and control groups (log-rank P ≤ 0.0001). Cox regression analysis indicated a significantly lower risk of AF recurrence in the SGLT2i group compared with the control group (HR 0.55, 95% CI [0.42, 0.71], P < 0.001). Figure 2a depicts the pooled KM curve for all four studies, while Fig. 2b presents the KM curve stratified by study, demonstrating the same symmetry as those reported in the original papers and validating the accuracy of our KM reconstruction. The Chi-squared test showed a significantly lower AF recurrence rate in the SGLT2i group (23.00%) compared with the control group (39.03%), P < 0.001 (Fig. 3).
3.4.2 SGLT2i Versus DPP4i
In the DPP4i group, there were 70 patients with 24 (34.28%) events (AF recurrence), while the SGLT2i group consisted of 71 patients with 10 (14.08%) events. The survival analysis based on reconstructed KM curves revealed a statistically significant difference between SGLT2i and DPP4i (log-rank P = 0.01) (Fig. 4). Cox regression analysis indicated a significantly lower risk of AF recurrence in the SGLT2i group compared with the DPP4i group (HR 0.39, 95% CI 0.19, 0.82, P = 0.013).
3.5 Direct Meta-analysis
After pooling the IPD, we performed a direct meta-analysis to confirm our results and explore potential heterogeneity in effect sizes resulting from different percentages of antidiabetic medications in the control group. Consistent with the reconstructed data, the meta-analysis revealed a statistically significant reduction in the risk of AF recurrence associated with SGLT2i compared with the control group (HR 0.57, 95% CI 0.44, 0.73), with no significant heterogeneity (I2 = 0%, P = 0.48) (Fig. 5). Notably, this effect size closely mirrored that obtained from the Cox regression model using the IPD from the four studies. We found consistent results when we examined SGLT2i versus DPP4i, with SGLT2i exhibiting a lower risk of AF recurrence (HR 0.41, 95% CI 0.24, 0.70) with no significant heterogeneity (I2 = 0%, P = 0.86) (Fig. 5).
3.6 Trial Sequential Analysis
A trial sequential analysis (TSA) conducted on AF recurrence using data from the four studies demonstrated that the cumulative Z-curve crossed the conventional boundary for benefit, as well as the trial sequential monitoring boundary (TSM boundary) with expected risk reduction set to 47%, an alpha of 5%, and power of 80%, indicating the robustness of the obtained evidence (Fig. S1).
4 Discussion
To our knowledge, this is the first meta-analysis conducted examining the efficacy of SGLT2i in AF recurrence after CA in patients with T2DM. We found that SGLT2i were associated with a statistically significant reduction in the risk of AF recurrence. Our results were consistent across the different statistical approaches used in our study, including the log-rank test, Cox regression, Chi-squared test, and direct meta-analysis. Our study is novel in focusing on patients with diabetes undergoing CA for AF and evaluating the role of SGLT2i. The expected differential treatment effect of SGLT2i stems from their cardiovascular benefits, including reducing inflammation, oxidative stress, and cardiac fibrosis. SGLT2i also improve glycemic control, reduce blood pressure, and promote weight loss, all aiding AF management. Given the higher cardiovascular risk in patients with type 2 diabetes, SGLT2i could significantly reduce AF recurrence and improve outcomes post-CA [27].
The efficacy of SGLT2i in reducing the risk of AF has been the subject of in-depth investigation in numerous prior studies. For example, a previous comprehensive meta-analysis encompassing 38,335 patients from 16 RCTs discovered that SGLT2i substantially diminished the risk of newly developing AF, overall mortality, incidents of heart failure, HbA1c level, body weight, and both systolic and diastolic blood pressure [6]. A different meta-analysis, which included 34 RCTs, suggested that SGLT2i were correlated with a decreased risk of newly developed atrial arrhythmias and sudden cardiac death in patients with T2DM or heart failure [23]. Furthermore, another comprehensive meta-analysis of 22 RCTs indicated that SGLT2i could potentially lower the risk of AF in patients with T2DM, heart failure, or chronic kidney disease [24]. However, they did not appear to decrease the risk of sudden cardiac death [24]. Collectively, these studies present strong evidence supporting the effectiveness of SGLT2i in mitigating the risk of AF in patients with T2DM. However, a key limitation across these studies is the absence of data at the individual patient level. As a result, these studies are unable to distinguish between new and recurrent cases of AF. Additionally, none of these studies investigated whether SGLT2i could confer benefits to patients with AF following CA.
The existing data regarding which antidiabetic drug is most effective in reducing the onset or recurrence of AF is both limited and inconclusive. A previous meta-analysis showed that DPP4i were associated with a lower risk of new-onset AF according to observational studies but not RCTs [25]. Metformin was observed to lower the risk of AF recurrence following CA in one study [9], whereas another study did not note any significant difference in AF recurrence [11]. Moreover, a previous retrospective study that compared SGLT2i with DPP4i found that SGLT2i were associated with a lower risk of newly developing AF. This aligns with our findings, with the distinction being that our study exclusively investigated the risk of AF recurrence following CA [26]. The aforementioned study also found that the effect was consistent regardless of age, gender, baseline HbA1c, cardiovascular disease, and chronic kidney disease [26]. Therefore, research on antidiabetic medications and their impact on AF recurrence is inconsistent, and further research is warranted. We believe that our study represents an important step in this topic, indicating that SGLT2i can reduce the likelihood of AF recurrence in individuals with T2DM following CA. In addition, some of the included SGLT2i in our study have been shown to have cardiovascular prevention effect. Therefore, this makes SGLT2i an appropriate treatment for this patient group, since they are at high risk of cardiovascular complications.
The mechanism by which SGLT2i positively impacts the cardiovascular system has been thoroughly explored in a myriad of foundational research studies. The antiarrhythmic benefits of SGLT2i are typically thought to stem from various direct and indirect mechanisms. Concerning direct mechanisms, arrhythmia has been associated with long-term systemic inflammation, oxidative stress, and fibrosis [27]. Studies suggest that dapagliflozin is associated with reduced reactive oxygen species and nitrogen species and decreased cardiac fibrosis [28]. Furthermore, dapagliflozin has been observed to significantly lower inflammatory markers and cytokines [29]. Similarly, canagliflozin has been found to lower serum interleukin-6 and tumor necrosis factor receptor-1 levels [30]. Previous studies have demonstrated that dapagliflozin inhibits cardiac fibrosis in post-myocardial infarction rat models [31], while other research has shown that SGLT2i reduces transforming growth factor-induced fibroblast activation and myocardial fibrosis [32]. Additionally, indirect mechanisms play a role, which includes the reduction of cardiac load and ventricular stress through blood pressure reduction and improvement in heart failure management. SGLT2i have also been found to suppress sympathetic nervous system activity and promote weight loss, contributing to an overall beneficial effect on the cardiovascular system [33].
4.1 Implications for Clinical Practice
In light of our findings, we suggest the potential benefits of adding SGLT2i to the treatment regimen of patients with T2DM who have a history of AF and have undergone CA. The inclusion of SGLT2i in the antidiabetic treatment algorithm may enable healthcare providers to improve the comprehensive management of AF and decrease the frequency of recurrent AF episodes. Furthermore, as SGLT2i have already demonstrated benefits in enhancing glycemic control, mitigating cardiovascular risks, and fostering weight loss in patients with T2DM, their added advantage in reducing AF recurrence makes them a compelling treatment option for this particular patient population [34]. Nonetheless, particular attention should be given to patients with persistent AF. The four studies we evaluated reported that persistent AF was significantly linked to a higher risk of AF recurrence, aligning with previous data [35].
4.2 Limitations and Implications for Future Research
Despite the impactful discoveries of this meta-analysis, we must recognize several limitations to enable a well-rounded interpretation of the results. Firstly, the number of studies incorporated in this meta-analysis was rather limited, which may affect the broad applicability of the findings. While five studies were included in the systematic review, only four of these studies were included in the meta-analysis, as one study did not report an outcome that was consistent with the other four studies. Even though the PRISMA guidelines guided the selection process, the small sample size could have hindered the overall statistical strength and possibly introduced bias. Secondly, the studies included in our analysis varied in design, encompassing one RCT and three cohort studies. Such inherent diversity could have influenced the overall effect estimate and introduced potential bias into the analysis. Thirdly, different SGLT2i were used in the included studies, potentially leading to varying effects on AF recurrence. Differences in pharmacokinetics and pharmacodynamics among these agents could have contributed to the unobserved heterogeneity in the results. Fourthly, although we conducted a rigorous methodological quality assessment, unaccounted confounding factors in the included studies could influence the results. The analysis did not account for variability in comorbidities, drug interactions, and individual patient characteristics. This is because using IPD from reconstructed KM curves does not allow us to access baseline characteristics such as BMI, weight, HbA1c, and others. More studies are required to take these variables into consideration and provide subgroup analysis. This will provide more robust evidence and help us understand how to maximize the benefit for these patients. Additonally, it would be interesting to examine the efficacy of SGLT2i against other antidiabetics. Our study could only capture the comparison of SGLT2i versus DDP4i because this is the only comparison that was made in the included studies. More studies with more comparisons are recommended.
Moreover, due to the observational nature of some of the included studies, there will be a proportion of patients who discontinued the drugs during follow-up. Additionally, there may be patients from the non-SGLT2i arm who were started on these drugs during follow-up. Furthermore, in the observational studies, it is possible that the fact that patients were prescribed SGLT2i indicates better clinical management since those patients were likely managed by physicians who were up-to-date with the newest guidelines [36]. This might be a confounding variable. Lastly, the duration of follow-up varied across the studies, which could affect the interpretation of the ongoing effectiveness of SGLT2i in reducing AF recurrence. A more extended follow-up period might have offered further insights into the long-term benefits of SGLT2i. Despite these limitations, this meta-analysis contributes valuable evidence to existing literature regarding the efficacy of SGLT2i in reducing AF recurrence after CA in patients with T2DM. Future research encompassing larger sample sizes and direct comparisons of different SGLT2i could offer more robust evidence concerning these agents’ effectiveness in managing AF in this demographic. Moreover, studies focusing on potential confounding factors and long-term outcomes could enhance our understanding of the advantages of SGLT2i in the clinical setting.
5 Conclusions
This meta-analysis suggests that SGLT2i are associated with a notable decrease in the recurrence of AF following CA in patients with T2DM. These findings highlight the potential therapeutic advantages of including SGLT2i as a supplementary treatment in this patient group. Nevertheless, further investigations and larger-scale studies are required to corroborate these results and to identify potential confounding factors.
References
Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case–control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108(1):56–62. https://doi.org/10.1016/J.AMJCARD.2011.03.004.
Dublin S, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25(8):853–8. https://doi.org/10.1007/S11606-010-1340-Y/FIGURES/2.
Kubota Y, Shimizu W. Clinical benefits of sodium-glucose cotransporter 2 inhibitors and the mechanisms underlying their cardiovascular effects. JACC Asia. 2022;2(3P2):287–93. https://doi.org/10.1016/J.JACASI.2022.03.009.
Mouffokes A, Soliman Y, Amer BE, Umar TP, Abdelazeem B. Abstract 13815: therapeutic effects of empagliflozin in diabetic patients after acute myocardial infarction: systematic review and meta-analysis with trial sequential analysis. Circulation (New York, N Y). 2023. https://doi.org/10.1161/circ.148.suppl_1.13815.
Zheng RJ, Wang Y, Tang JN, Duan JY, Yuan MY, Zhang JY. Association of SGLT2 inhibitors with risk of atrial fibrillation and stroke in patients with and without type 2 diabetes: a systemic review and meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2022;79(2):E145–52. https://doi.org/10.1097/FJC.0000000000001183.
Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020. https://doi.org/10.1186/S12933-020-01105-5.
Jaïs P, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118(24):2498–505. https://doi.org/10.1161/CIRCULATIONAHA.108.772582.
Guckel D, et al. The effect of diabetes mellitus on the recurrence of atrial fibrillation after ablation. J Clin Med. 2021;10(21):4863. https://doi.org/10.3390/JCM10214863/S1.
Deshmukh A, et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32(5):1232–9. https://doi.org/10.1111/JCE.14954.
Gu J, et al. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. EP Europace. 2011;13(9):1256–61. https://doi.org/10.1093/EUROPACE/EUR131.
Wang A, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2. 2020;1(3):180. https://doi.org/10.1016/J.HROO.2020.04.006.
Kubota Y, Shimizu W. Clinical benefits of sodium-glucose cotransporter 2 inhibitors and the mechanisms underlying their cardiovascular effects. JACC Asia. 2022;2(3):287–93. https://doi.org/10.1016/J.JACASI.2022.03.009.
Luo F, et al. Effect of dapagliflozin on the outcome of radiofrequency catheter ablation in patients with type 2 diabetes mellitus and atrial fibrillation. Cardiovasc Drugs Ther. 2022. https://doi.org/10.1007/s10557-022-07368-2.
Liu HT, Wo HT, Chang PC, Lee HL, Wen MS, Chou CC. Long-term efficacy of sodium-glucose cotransporter 2 inhibitor therapy in preventing atrial fibrillation recurrence after catheter ablation in type 2 diabetes mellitus patients. Heliyon. 2023;9(6): e16835. https://doi.org/10.1016/j.heliyon.2023.e16835.
Kishima H, Mine T, Fukuhara E, Kitagaki R, Asakura M, Ishihara M. Efficacy of sodium-glucose cotransporter 2 inhibitors on outcomes after catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2022;8(11):1393–404. https://doi.org/10.1016/j.jacep.2022.08.004.
Abu-Qaoud MR, et al. Impact of SGLT2 Inhibitors on atrial fibrillation recurrence after catheter ablation in patients with type-2-diabetes. JACC Clin Electrophysiol. 2023. https://doi.org/10.1016/j.jacep.2023.06.008.
Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):1–22. https://doi.org/10.1186/s12874-021-01308-8.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2020. https://doi.org/10.1136/BMJ.N71.
Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. https://doi.org/10.1136/BMJ.L4898.
Wells D, Shea GA, O’Connel B, et al. The Newcastle–Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses; 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [cited 19 Oct 2009].
Greco A, Capodanno D. Trial sequential analysis methodology for interpreting meta-analytical findings. Eur J Intern Med. 2024. https://doi.org/10.1016/j.ejim.2023.12.029.
Zhao Z, et al. Impact of sodium-glucose cotransporter 2 inhibitor on recurrence after catheter ablation for atrial fibrillation in patients with diabetes: a propensity-score matching study and meta-analysis. J Am Heart Assoc. 2023. https://doi.org/10.1161/JAHA.123.031269.
Fernandes GC, et al. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18(7):1098–105. https://doi.org/10.1016/J.HRTHM.2021.03.028.
Li HL, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021. https://doi.org/10.1186/S12933-021-01293-8.
Zhang Z, et al. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017. https://doi.org/10.1186/S12872-017-0531-4.
Ling AWC, et al. The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors. Cardiovasc Diabetol. 2020;19(1):1–12. https://doi.org/10.1186/s12933-020-01162-w.
Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840(9):2709–29. https://doi.org/10.1016/J.BBAGEN.2014.05.017.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. https://doi.org/10.1016/J.FREERADBIOMED.2017.01.035.
Zhang N, Feng B, Ma X, Sun K, Xu G, Zhou Y. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019. https://doi.org/10.1186/S12933-019-0914-1.
Heerspink HJL, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–66. https://doi.org/10.1007/S00125-019-4859-4.
Kang S, et al. Direct effects of empagliflozin on extracellular matrix remodelling in human cardiac myofibroblasts: novel translational clues to explain EMPA-REG OUTCOME results. Can J Cardiol. 2020;36(4):543–53. https://doi.org/10.1016/J.CJCA.2019.08.033.
Tanaka H, et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020. https://doi.org/10.1186/S12933-019-0985-Z.
Wu J, et al. Antiarrhythmic effects and mechanisms of sodium-glucose cotransporter 2 inhibitors: a mini review. Front Cardiovasc Med. 2022. https://doi.org/10.3389/FCVM.2022.915455.
Baruah MP, Makkar BM, Ghatnatti VB, Mandal K. Sodium glucose co-transporter-2 inhibitor: benefits beyond glycemic control. Indian J Endocrinol Metab. 2019;23(1):140. https://doi.org/10.4103/IJEM.IJEM_160_17.
Darby AE. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation. 2016;9(1):1427. https://doi.org/10.4022/jafib.1427.
Calvert P, Gupta D, Proietti R. SGLT2 inhibitors and catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2023. https://doi.org/10.1016/j.jacep.2023.06.017.
Acknowledgments
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Y.S. conceived the idea. B.E. and A.M. designed the research workflow. M.H. and A.M. searched the databases. M.K., M.H., and A.M. screened the retrieved records, extracted relevant data, and assessed the quality of evidence. Y.S. performed the analysis. All authors contributed to writing and reviewing the manuscript. B.A. and H.H. supervised the project and approved the final manuscript version. All authors have read and agreed to the final version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Funding
We received no funding for this study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The data utilized in this study can be provided in a Microsoft Excel format upon reasonable request directed to the first author (Soliman).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Soliman, Y., Abuelazm, M., Amer, B.E. et al. Impact of SGLT2 Inhibitors on Atrial Fibrillation Recurrence after Catheter Ablation in Type 2 Diabetes Mellitus: A Meta-Analysis of Reconstructed Kaplan–Meier Curves with Trial Sequential Analysis. Am J Cardiovasc Drugs 24, 629–640 (2024). https://doi.org/10.1007/s40256-024-00661-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-024-00661-5