Abstract
Background
The cytochrome P450 (CYP) 2C9 enzyme plays a role in the metabolization of clopidogrel. Carriage of a CYP2C9 loss-of-function (LoF) allele has been associated with attenuated pharmacokinetics, leading to a diminished pharmacodynamic response and increased risk for developing stent thrombosis in patients treated with clopidogrel.
Methods
In this study, we aimed to determine the effect of the CYP2C9*2 and *3 LoF alleles on thrombotic events. Therefore, a post hoc analysis was performed in 878 patients with available CYP2C9 genotype status included in the POPular Genetics and POPular Age trials, which enrolled patients with ST-elevation myocardial infarction and non-ST-elevation myocardial infarction, respectively. The primary thrombotic outcome was a composite of cardiovascular death, myocardial infarction or stroke.
Results
A total of 526 (60%) patients were CYP2C9 LoF allele noncarriers and 352 (40%) were CYP2C9 LoF allele (*2 or *3) carriers. After correction for differences in baseline characteristics, there were no significant differences between CYP2C9 LoF allele carriers and noncarriers for the combined thrombotic outcome (6.3% vs. 5.9%, hazard ratio 1.16 [0.67–2.0], p = 0.60), or the individual thrombotic outcomes. Moreover, no differences were seen in the event rates for clinically relevant bleeding (Bleeding Academic Research Consortium [BARC] 2–5 bleeding) as well as major bleeding (BARC 3 or 5 bleeding).
Conclusions
Carriers of a CYP2C9 *2 or *3 LoF allele presenting with acute coronary syndrome and treated with clopidogrel did not have an increased risk for thrombotic events compared with noncarriers. Given the limited number of poor metabolizers, no firm conclusions could be drawn with regard to the thrombotic risk for patients carrying two CYP2C9 LoF alleles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients who carried a cytochrome P450 (CYP) 2C9 loss-of-function allele did not have an increased risk for thrombotic events when treated with clopidogrel compared with noncarriers. |
It is not necessary to test all patients for the CYP2C9 polymorphisms when starting treatment with clopidogrel. |
1 Introduction
In patients with chronic coronary syndrome (CCS) undergoing percutaneous coronary intervention (PCI), dual antiplatelet therapy (DAPT) consisting of aspirin and clopidogrel represents the cornerstone of medical therapy to prevent thrombotic complications [1]. In patients with acute coronary syndrome (ACS), use of the more potent P2Y12 inhibitors prasugrel or ticagrelor is preferred [2]. However, in the current guidelines, guided or unguided de-escalation of potent P2Y12 inhibition, by switching from ticagrelor or prasugrel to clopidogrel, may be considered an alternative DAPT strategy (class IIb, level of evidence A) in patients with ACS deemed unsuitable for potent platelet inhibition (e.g. those with high bleeding risk) [3]. Treatment with clopidogrel is subject to large interindividual variability. During the two-step conversion of clopidogrel to its active metabolite, multiple cytochrome P450 (CYP) enzymes play a part (CYP2C19, CYP3A4/5, CYP1A2, CYP2B6 and CYP2C9) [4], however CYP2C19 is the main contributor in this process. As a consequence, carriers of a CYP2C19 loss-of-function (LoF) allele have a reduced antiplatelet response to clopidogrel and are at higher risk for thrombotic events when compared with noncarriers [5, 6]. Therefore, guided de-escalation to clopidogrel can be done by CYP2C19 genotyping [7]. However, there are other CYP enzyme polymorphisms involved in the metabolization of clopidogrel that can also affect antiplatelet response to clopidogrel. The CYP2C9 enzyme, which is an important member of the CYP enzyme family, also plays a role in the metabolization of clopidogrel. Of all known variants of the CYP2C9 gene, CYP2C9*2 (rs1799853) and CYP2C9*3 (rs1057910) are the most common LoF variants with reduced enzymatic activity [8, 9]. Carriage of a CYP2C9 LoF allele has been associated with a lower exposure to clopidogrel active metabolite and a diminished pharmacodynamic response [10, 11]. The CYP2C9*3 LoF variant has even been associated with a 2.4-fold increased risk for developing stent thrombosis (ST) in patients treated with clopidogrel after PCI [12]. Thus, the presence of a CYP2C9 LoF allele may affect an individual’s response to clopidogrel and therefore potentially impact clinical outcomes. However, there is a lack of data regarding the different CYP2C9 polymorphisms and their prognostic impact in patients treated with clopidogrel. In this post hoc analysis, our aim was to assess the effect of the CYP2C9 LoF alleles on clopidogrel treatment in ACS patients and investigate whether these alleles influence the risk of occurrence of thrombotic and/or bleeding events using data from two large randomized controlled trials—POPular Genetics and POPular Age [13, 14].
2 Methods
2.1 Study Design and Population
The design and results of the POPular Genetics and POPular Age trials have been published previously. In brief, the POPular Genetics trial was an open-label, assessor-blinded, randomized controlled trial [13]. Between 2012 and 2018, 2488 patients with ST-elevation myocardial infarction (STEMI) undergoing primary PCI aged 21 years and older were included. Within 48 hours of primary PCI, patients were randomized to either a standard treatment arm (treatment with ticagrelor or prasugrel for 1 year) or to the genotype-guided arm (treatment adjustment after rapid CYP2C19 genetic testing). In the genotype-guided arm, patients carrying a CYP2C19*2 or *3 LoF allele were treated with ticagrelor or prasugrel, while noncarriers (*1/*1) were treated with clopidogrel. The POPular AGE trial was an open-label, assessor-blinded, randomized controlled trial performed in 12 centers in The Netherlands [14]. Between 2013 and 2018, 1002 patients with non-STEMI (NSTEMI) and unstable angina aged 70 years and older were included. Patients were randomized to either treatment with clopidogrel or treatment with ticagrelor or prasugrel on top of standard care. The follow-up duration was 12 months in both trials. An Institutional Review Board approved the trials and all patients provided written informed consent. This analysis included all patients in whom the CYP2C9 gene was analyzed.
2.2 Data Collection
In both studies, CYP2C9 genotyping was retrospectively performed using blood samples, which were analyzed by LGC Biosearch Technologies (Hoddesdon, UK) using a KASP genotyping assay. In the POPular AGE trial, blood samples were collected in three participating hospitals and were therefore not available for all patients. In the POPular Genetics trial, CYP2C19 genotyping was performed with the use of the TaqMan StepOnePlus assay at a central laboratory (St. Antonius Hospital, Nieuwegein, The Netherlands) or with an on-site point-of-care Spartan RX device (Spartan Bioscience). In the POPular AGE trial, CYP2C19 genotyping was performed by LGC Biosearch Technologies using a KASP genotyping assay.
2.3 Outcomes and Definitions
Cardiovascular events consisted of all-cause death, cardiovascular death, myocardial infarction (MI), stroke, ST, target vessel revascularization (TVR), unstable angina, transient ischemic attack (TIA) and any bleeding requiring medical attention classified according to the PLATelet inhibition and patient Outcomes (PLATO) bleeding classification, as well as the Bleeding Academic Research Consortium (BARC) bleeding criteria, at 1-year follow-up. Clinically relevant bleeding was defined as PLATO minor and major bleeding or BARC 2–5 bleeding. The primary thrombotic outcome was a composite of death from cardiovascular causes, MI, or stroke. The outcome definitions were identical to the definitions used in both main trials, in which a blinded event committee adjudicated all adverse clinical events.
2.4 Statistical Analysis
This analysis was not prospectively powered and the sample size is based on the number of patients in the original trials of whom the CYP2C9 genetic profile was available. In this analysis, we compared the thrombotic event rate between carriers and noncarriers of a CYP2C9 LoF allele (*2 or *3) treated with clopidogrel (dominant model). For survival analyses, patients were also divided into three groups on the basis of their phenotype: extensive metabolizers (CYP2C9*1/*1 homozygotes), intermediate metabolizers (CYP2C9*1/*2 and CYP2C9*1/*3 heterozygotes) and poor metabolizers (CYP2C9*2/*2, CYP2C9*2/*3 and CYP2C9*3/*3). Variables are presented as number (percentages) and mean ± standard deviation (SD), or median and interquartile range. Time-to-event curves were constructed using the Kaplan–Meier method. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated using Cox proportional hazard models and adjusted for baseline differences by including all variables with a p value <0.05. p values <0.05 were considered statistically significant. Chi-square analysis was used to test the deviations of genotype distribution from the Hardy–Weinberg equilibrium.
3 Results
The POPular Genetics and POPular Age trials included a total of 2488 and 1002 patients, respectively. A flowchart of the selection of patients from these two trials with CYP2C9 status is presented in Fig. 1. The CYP2C9 genotype was available in 2062 (83%) patients in the POPular Genetics trial and 465 (46%) patients in the POPular Age trial, resulting in a total population of 2527 patients with a known CYP2C9 genotype. From this cohort, 878 (35%) patients were treated with clopidogrel, of whom 526 (60%) were noncarriers of a CYP2C9 LoF allele and 352 (40%) were CYP2C9 LoF allele carriers. The baseline characteristics are shown in Table 1. Between CYP2C9 LoF allele carriers and noncarriers, all variables were well balanced, except for a higher frequency of CYP2C19 LoF carriers (5.1% vs. 11.6%, p = 0.001) and NSTEMI diagnosis at discharge (17.9% vs. 23.6%, p = 0.044) in the CYP2C19 LoF carrier group. Genotype distributions were in Hardy–Weinberg equilibrium and frequencies were similar to those in other studies of Caucasian participants (Fig. 2) [10, 12].
Oversight of the different allele frequencies of both the CYP2C9 *2 and *3 genotypes. a Allele frequencies separated for the CYP2C9 *2 and *3 LoF alleles. The table shows the total allele frequencies for both the CYP2C9 *2 and *3 LoF alleles. b CYP2C9 frequencies according to their phenotype (extensive metabolizers, intermediate metabolizers or poor metabolizers). AF allele frequency, CYP cytochrome P450, LoF loss-of-function, SNP single nucleotide polymorphism
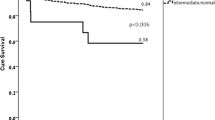
The occurrence of cardiovascular events was comparable in both carriers and noncarriers of a CYP2C9 LoF allele (Fig. 3a; Table 2). No significant differences were seen between carriers and noncarriers of a CYP2C9 LoF allele for the combined thrombotic outcome (6.3% vs. 5.9%, HR 1.16 [0.67–2.02], p = 0.60) or the individual thrombotic outcomes, after correction for CYP2C19 LoF allele carrier status and diagnosis at discharge. Event rates for thrombotic outcomes were numerically higher in poor metabolizers compared with extensive metabolizers (9.5% vs. 5.9%, HR 1.93 [0.67–5.54], p = 0.22). Unfortunately, the number of poor metabolizers was very low (n = 42). When specifically assessing the different CYP2C9 polymorphisms, there was no statistically significant difference in the thrombotic outcome between CYP2C9 *3 carriers and noncarriers (7.5% vs. 5.8%, HR 1.42 [0.71–2.84], p = 0.32) or between CYP2C9 *2 carriers and noncarriers (6.2% vs. 6.0%, HR 1.13 [0.62–2.07], p = 0.68). No differences were seen in the event rates for clinically relevant bleeding (BARC 2–5 bleeding: 12.5% vs. 14.8%, HR 0.90 [0.62–1.30], p = 0.57; PLATO minor and major bleeding: 11.4% vs. 13.9%, HR 0.86 [0.58–1.27], p = 0.45), or for major bleeding (BARC 3 or 5 bleeding: 3.7% vs. 3.8%, HR 1.08 [0.53–2.19], p = 0.83; PLATO major bleeding: 2.8% vs. 3.2%, HR 0.93 [0.42–2.05], p = 0.86) (Fig. 3b; Table 2).
Outcomes of clopidogrel-treated patients with regard to the composite thrombotic and bleeding outcome in CYP2C9 LoF carriers compared with noncarriers. Kaplan–Meier curves for a the thrombotic outcome, consisting of cardiovascular death, myocardial infarction and stroke, and b BARC 2–5 bleeding. BARC Bleeding Academic Research Consortium, CYP cytochrome P450, LoF loss-of-function
4 Discussion
In this post hoc analysis investigating the prognostic impact of the CYP2C9 polymorphisms on cardiovascular outcomes in patients treated with clopidogrel for ACS (POPular Genetics and POPular Age trials), the main finding is that there were no differences in the risk for thrombotic events between CYP2C9 LoF carriers and noncarriers. This was also the case when specifically assessing the impact of the CYP2C9 *3 allele on thrombotic events.
The CYP2C9 enzyme plays an important role in the metabolization of about 15% of clinically administered drugs. Several studies have shown that the presence of a CYP2C9 polymorphism can have clinical implications in different antithrombotic treatments. One of the most significant clinical impacts of CYP2C9 polymorphisms is the metabolism of Vitamin K antagonists (VKA, such as warfarin and acenocoumarol), with an approximately fivefold stronger anticoagulant effect. Poor metabolizers with a CYP2C9 LOF variant need lower doses of VKA to attain an adequate response and are more prone to adverse effects such as bleeding [15, 16]. Next to VKAs, CYP2C9 also plays a role in the metabolization of aspirin and CYP2C9 polymorphisms have been associated with a higher risk for adverse effects and aspirin intolerance [17, 18].
CYP2C9 only plays a role in the second metabolization step in the activation of clopidogrel [5]. In addition, the presence of a CYP2C9 LoF allele has been associated with decreased exposure to the active metabolite of clopidogrel and a diminished pharmacodynamic response. In the study by Harmsze et al. regarding patients undergoing elective coronary stent implantation, carriers of the CYP2C9*3 LoF allele showed overall higher on-clopidogrel platelet reactivity and an increased risk for a poor response to clopidogrel, indicating that the genetic variant CYP2C9*3 plays an important role in response to clopidogrel [11]. However, in healthy subjects, there is more controversy between the association of the CYP2C9 LoF alleles and the effect of clopidogrel. While Brandt et al. showed that carriers of the CYP2C9 *2 or *3 allele had decreased exposure to the active metabolite of clopidogrel and were more often poor responders, Mega et al. did not find any association with CYP2C9 LoF alleles and the pharmacokinetic and pharmacodynamic response to clopidogrel [5, 10]. Moreover, in line with our results, Mega et al. observed no significant associations between the CYP2C9 genotype and thrombotic events in a cohort of 1477 patients treated with clopidogrel; however, they assessed neither poor responder status nor the effect of the individual CYP2C9*2 and *3 polymorphisms in their analysis. Compared with the population in the study by Mega et al., the number of carriers of a CYP2C9 LoF allele was higher in our study population, although our overall population was slightly smaller. In contrast to their analysis, we did assess the impact of the different CYP2C9 LoF alleles separately. Numerically, the thrombotic event rates were higher in carriers of a CYP2C9*3 allele (7.5% vs. 5.8%), while this was not the case in carriers of the CYP2C9*2 allele (6.2% vs. 6.0%). As these differences were not statistically significant, no firm conclusions can be drawn from these results. However, this observation is in line with a previous case-control study by Harmsze et al. showing that the CYP2C9 *3 LoF allele in particular was associated with an increased risk for ST, while the CYP2C9 *2 LoF allele was not [12].
As previous studies have shown that CYP2C9 LoF allele carriers have decreased exposure to the active metabolite of clopidogrel, it can be hypothesized that this could lead to a lower risk for adverse effects such as bleeding. Nonetheless, we found no differences in bleeding rates between the two groups that could substantiate this hypothesis.
Overall, our results suggest that there is no increased risk for thrombotic events in patients who carry a CYP2C9 *2 or CYP2C9 *3 LoF allele. This is reassuring as it implicates that it is not necessary to test for the CYP2C9 polymorphisms when starting treatment with clopidogrel. Currently, CYP2C19 remains the most important CYP enzyme in the metabolization of clopidogrel, affecting the pharmacokinetics, pharmacodynamic response and risk on thrombotic events in patients with a CYP2C19 LoF allele. In recent years, there has been growing evidence in favor of a CYP2C19 genotype-guided treatment strategy when prescribing DAPT in patients with ACS, implicating that tailoring the P2Y12 inhibitor based on a patient’s CYP2C19 genotype can improve cardiovascular outcomes [13, 19, 20]. In particular, the POPular Genetics trial showed that by applying a genotype-guided de-escalation strategy, 61% of patients could be treated with clopidogrel instead of ticagrelor, which resulted in a substantially lower risk of bleeding events without any evidence for an increase in ischemic events [13].
4.1 Limitations
There are limitations in this study. First, this post hoc analysis was dependent on the available CYP2C9 genotype data of the POPular Genetics and POPular Age trial. As a result, it was not prospectively powered to detect a difference in the cardiovascular outcomes. Our results should therefore be interpreted in light of the limited sample size. Second, in the POPular Genetics trial, patients were randomized between a CYP2C19 genotype-guided treatment strategy versus conventional treatment. Therefore, the CYP2C19 genotype was not normally distributed over the patients treated with clopidogrel, which is also reflected in the baseline table. However, in our analyses, we adjusted for the carrier status of the CYP2C19 LoF allele to account for this unequal distribution. Finally, due to the low prevalence of the poor metabolizer phenotype, we used a dominant model in our primary analysis, assuming that having one or more copies of the CYP2C9*2 or *3 allele increased the thrombotic risk compared with the *1/*1 genotype. Therefore, a possible effect limited to CYP2C9 poor metabolizers might have been underestimated or missed.
5 Conclusion
Carriage of the CYP2C9 *2 or *3 LoF alleles did not increase the risk for thrombotic events in patients presenting with ACS treated with clopidogrel. Due to the limited number of poor metabolizers, further studies should be conducted in a larger cohort treated with clopidogrel to be able to definitely rule out an association between CYP2C9 poor metabolizers and thrombotic risk.
References
Knuuti J, Wijns W, Flachskampf FA, Gohlke H, Grove EL, James S, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77.
Valgimigli M, Bueno H, Byrne RA, Collet J-P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European. Eur Heart J. 2018;39:213–60.
Collet J-P, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42:1289–367. https://academic.oup.com/eurheartj/article/42/14/1289/5898842
Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–9.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–62.
Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30.
Ancrenaz V, Daali Y, Fontana P, Besson M, Samer C, Dayer P, et al. Impact of genetic polymorphisms and drug–drug interactions on clopidogrel and prasugrel response variability. Curr Drug Metab. 2010;11:667–77.
Xie HG, Prasad HC, Kim RB, Stein CM. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev. 2002;54:1257–70.
Kirchheiner J, Brockmöller J. Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin Pharmacol Ther. 2005;77(1):1–16.
Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–36.
Harmsze A, Van Werkum JW, Bouman HJ, Ruven HJ, Breet NJ, Ten Berg JM, et al. Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenet Genom. 2010;20:18–25.
Harmsze AM, Van Werkum JW, Ten Berg JM, Zwart B, Bouman HJ, Breet NJ, et al. CYP2C19*2 and CYP2C9*3 alleles are associated with stent thrombosis: a case-control study. Eur Heart J. 2010;31:3046–53.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, Van’t Hof AWJ, Van Der Harst P, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621–31.
Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–81.
Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnetTM systematic review and meta-analysis. Genet Med. 2005;7(2):97–104.
Schalekamp T, Oosterhof M, Van Meegen E, Van Der Meer FJM, Conemans J, Hermans M, et al. Effects of cytochrome P450 2C9 polymorphisms on phenprocoumon anticoagulation status. Clin Pharmacol Ther. 2004;76:409–17.
Agundez J, Martinez C, Perez-Sala D, Carballo M, Torres M, Garcia-Martin E. Pharmacogenomics in aspirin intolerance. Curr Drug Metab. 2010;10:998–1008.
Palikhe NS, Kim SH, Nam YH, Ye YM, Park HS. Polymorphisms of aspirin-metabolizing enzymes CYP2C9, NAT2 and UGT1A6 in aspirin-intolerant urticaria. Allergy Asthma Immunol Res. 2011;3:273–6.
Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv. 2021;14:739–50.
Beitelshees AL, Thomas CD, Empey PE, Stouffer GA, Angiolillo DJ, Franchi F, et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J Am Heart Assoc. 2022;11:e024159. https://doi.org/10.1161/JAHA.121.024159.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The POPular Genetics (NCT01761786) and POPular Age trials (NCT02317198) were supported by grants from the Netherlands Organization for Health Research and Development (ZonMW). The authors are solely responsible for designing and conducting this study, conducting all study analyses, and drafting and editing the manuscript and its final contents.
Conflict of interest
Jurriën M. ten Berg reports institutional grants from the Netherlands Organization for Health Research and Development, a Dutch government institution called ZonMw, and AstraZeneca, as well as personal fees from AstraZeneca, Boehringer Ingelheim, Bayer, Ferrer, Pfizer, and Merck, outside the submitted work. Wout W.A. van den Broek, Nabil Mani, and Jaouad Azzahhafi declare no conflicts of interest.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the Institutional Review Board (MEC-U). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent to participate
All patients provided written informed consent.
Authors’ contributions
JtB designed the studies and WB performed all the statistical analyses. All authors interpreted the data, and WB and JtB wrote the paper. All authors critically reviewed the manuscript.
Consent for publication
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
van den Broek, W.W.A., Mani, N., Azzahhafi, J. et al. CYP2C9 Polymorphisms and the Risk of Cardiovascular Events in Patients Treated with Clopidogrel: Combined Data from the POPular Genetics and POPular AGE Trials. Am J Cardiovasc Drugs 23, 165–172 (2023). https://doi.org/10.1007/s40256-022-00565-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-022-00565-2