Abstract
Patients surviving an acute coronary syndrome (ACS) remain at increased risk of ischemic events long term. This paper reviews current evidence and guidelines for oral antiplatelet therapy for secondary prevention following ACS, with respect to decreased risk of ischemic events versus bleeding risk according to individual patient characteristics and risk factors. Specifically, data are reviewed from clinical studies of clopidogrel, prasugrel, ticagrelor and vorapaxar, as well as the results of systematic reviews and meta-analyses looking at the benefits and risks of oral antiplatelet therapy, and the relative merits of shorter versus longer duration of dual antiplatelet therapy, in different patient groups.
Similar content being viewed by others
Patients surviving an acute coronary syndrome (ACS) remain at increased risk of ischemic events long term. |
The availability of new antiplatelet agents and extended or combination therapy has increased the options for secondary prevention among ACS patients. |
1 Introduction
Patients with a history of acute coronary syndrome (ACS) remain at increased risk of ischemic events long term [1,2,3]. Data from the Global Registry of Acute Coronary Events (GRACE) showed that more than half (53.6%) of ACS patients were re-hospitalized at least once during the 5-year follow-up period after discharge [3]. During the immediate 2 years after ACS, 7.1% of patients died, 6.3% experienced heart failure, and 4.4% experienced reinfarction, despite treatment aimed at secondary prevention [4]. In another global registry, Reduction of Atherothrombosis for Continued Health (REACH), almost a fifth of patients with a prior myocardial infarction (MI) either died or experienced another MI or a stroke over the following 4 years, with the greatest risk in those who had had an event within the year prior to enrollment [1]. In recent years, the outlook for ACS patients has improved with the expansion of available options for antithrombotic treatment [5]. This narrative review provides a critical discussion based on the author’s review of the medical literature concerning current oral antiplatelet therapy for secondary prevention following ACS with respect to individual patient characteristics and risk factors.
2 Platelet Activation
Platelets play a pivotal role in the pathogenesis of ACS. While activation of circulating platelets is essential for normal hemostasis in response to vascular injury, their activation and aggregation in the context of atherosclerotic plaque rupture or erosion promote pathological thrombus formation [6]. Atherosclerotic plaque and thrombi may occlude the blood vessels, thereby blocking the supply of oxygen to the tissues and resulting in an ischemic event. When the coronary arteries are affected, this can result in stable or unstable angina, depending on the degree and nature of the blockage; if the ischemia is severe, the outcome is MI and necrosis.
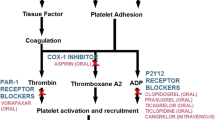
Multiple cellular pathways participate in the activation and aggregation of platelets at the site of endothelial disruption, and represent pharmacological targets for the acute and long-term treatment of atherothrombosis (Fig. 1) [5]. Secondary prevention strategies for ACS patients currently focus on the inhibition of three key platelet activation pathways: thromboxane A2 (TXA-2) generation via cyclooxygenase-1 (COX-1); adenosine diphosphate (ADP)-mediated activation of the P2Y12 receptor; and thrombin-mediated activation of protease-activated receptor-1 (PAR-1).
3 Oral Antiplatelet Agents
Table 1 provides a summary of the key attributes of the oral antiplatelet agents described in this section.
3.1 Aspirin
The benefit of aspirin therapy for secondary prevention of ischemic events in patients at high risk for atherothrombosis is well established [7]. Aspirin irreversibly acetylates COX-1, inhibiting formation of the pro-thrombotic mediator TXA-2 from arachidonic acid. Its antiplatelet effects occur rapidly, and it takes 3–4 days for complete recovery of platelet aggregation after stopping treatment [8].
Aspirin remains a first-line, foundation treatment for prevention of ischemic events after ACS, and a daily maintenance dose of 75–100 mg is recommended indefinitely [9, 10]. Lower aspirin doses are preferred because higher doses (≥ 160 mg) are usually associated with increased bleeding risk without an improvement in ischemic outcomes [7, 11, 12]. As aspirin cannot prevent platelet activation via other pathways, combination therapy with another oral antiplatelet agent is usually recommended, and the combined use of aspirin and P2Y12 inhibitors has been shown to provide additive inhibition of platelet activation [5, 13]. Aspirin resistance, i.e., a lower than normal platelet inhibitory effect, has been reported in some patient populations, and may be addressed by increasing the frequency of intake and/or combination with other antiplatelet agents [14].
3.2 Clopidogrel
Ticlopidine and clopidogrel represent the first and second generation of P2Y12 inhibitors, respectively, and both belong to the thienopyridine class of antiplatelet drugs that selectively and irreversibly prevent binding of ADP to the P2Y12 receptor. While effective as an antiplatelet agent, the use of ticlopidine is associated with potentially serious adverse effects, including bone marrow suppression [15]; therefore, clopidogrel is currently the most widely used P2Y12 inhibitor.
Clopidogrel is a prodrug, requiring hepatic conversion via cytochrome (CYP) P450 enzymes to produce an active metabolite. This means it can take up to 8 h after a loading dose of clopidogrel to achieve significant platelet inhibitory effects [16]. Clopidogrel responsiveness may be diminished by concomitant administration of drugs that competitively inhibit its activation by CYP enzymes, such as proton pump inhibitors [17]. As binding of the clopidogrel metabolite to the P2Y12 receptor is irreversible, restoration of platelet function is delayed until the body produces new platelets. Therefore, clopidogrel should be discontinued at least 5 days prior to elective surgery [9].
Dual antiplatelet therapy, predominantly with clopidogrel and aspirin, has been the backbone of secondary prevention of recurrent ischemic events in ACS patients for over a decade. The pivotal Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial demonstrated a 20% relative risk reduction in major adverse cardiovascular (CV) events (MACE) (death from CV causes, non-fatal MI, or stroke) in non-ST-elevation (NSTE)-ACS patients treated with clopidogrel plus aspirin versus aspirin alone for 12 months following ACS [18]. The benefit of clopidogrel was maintained from 2 h post-administration to the end of follow-up and was largely accounted for by a reduction in the risk of non-fatal MI. Subsequent studies confirmed the secondary prevention benefit of clopidogrel plus aspirin in patients with ST-elevation MI (STEMI) managed with fibrinolytics and in the setting of elective percutaneous coronary intervention (PCI) [19, 20].
However, it is well recognized that there is a considerable degree of inter-individual variability in response to clopidogrel as a result of multiple factors, including age, diabetes mellitus, drug–drug interactions, and genetic polymorphisms (particularly those affecting CYP2C19, the principal enzyme group involved in its metabolic activation) [21]. A review of 15 prospective studies noted that approximately 25% of patients were clopidogrel non-responders according to ADP aggregation testing; they exhibited high on-treatment platelet reactivity (HPR), which was associated with a 3.5-fold greater risk of recurrent ischemic events [22]. This review is supported by data from the National Institutes of Health (NIH)-funded Implementing GeNomics In pracTicE (IGNITE) network study, which found that in patients with a non-functional allele, the risk of MACE was significantly greater with clopidogrel compared with other antiplatelet therapies [23]. Consequently, the clopidogrel prescribing information contains a boxed warning about higher CV event rates in poor metabolizers [24]. The third-generation P2Y12 inhibitors, prasugrel and ticagrelor, were developed with the aim of addressing the slow onset and heterogeneous platelet inhibiting properties of clopidogrel, and the Clinical Pharmacogenetics Implementation Consortium and the institutions involved in the IGNITE project collectively recommend that patients with poor or intermediate metabolizer phenotypes should be given treatment other than clopidogrel, such as prasugrel or ticagrelor [23]. It should be noted that a clinical study exploring CYP2C19 genotype-guided therapy after PCI is ongoing and these recommendations are based on clinical opinion and experience rather than clinical trial evidence.
3.3 Newer P2Y12 Inhibitors
3.3.1 Prasugrel
Like clopidogrel, prasugrel is a thienopyridine and, therefore, blocks ADP binding to the P2Y12 receptor irreversibly. It is also a prodrug, requiring metabolic activation, but has a faster onset of action than clopidogrel [25]. It is recommended that prasugrel is stopped at least 7 days prior to elective coronary artery bypass graft (CABG) surgery (class I recommendation), but shorter delays may be reasonable in patients referred for urgent CABG (class IIb recommendation) [9].
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38) established prasugrel as superior to clopidogrel for the secondary prevention of recurrent ischemic events following ACS, in patients managed with PCI [26]. Dual antiplatelet therapy with prasugrel and aspirin reduced the incidence of death from CV causes, non-fatal MI, or non-fatal stroke by 19% at 15 months, compared with clopidogrel and aspirin (hazard ratio [HR] 0.81, 95% confidence interval [CI] 0.73–0.90; p < 0.001) (Table 2). Rates of stent thrombosis were also lower for prasugrel plus aspirin compared with clopidogrel plus aspirin (1.1 vs. 2.4%; p < 0.001), but rates of TIMI-defined non–CABG-related major bleeding were significantly greater in the prasugrel-treated versus clopidogrel-treated group, including life-threatening and fatal bleeding (Table 2). However, considering both ischemic and bleeding events, the net clinical benefit was in favor of prasugrel (HR 0.87, 95% CI 0.79–0.95; p = 0.004). A subgroup analysis of TRITON-TIMI 38 identified an excess of intracranial bleeding with prasugrel treatment in patients with a prior stroke or transient ischemic attack (TIA), which resulted in net harm. There was also no net benefit of prasugrel in patients aged 75 years or older or those weighing less than 60 kg. As a result of these observations, the prasugrel prescribing information contains a boxed warning against its use in patients with active pathological bleeding or a history of TIA or stroke, and provisos concerning its use in older and lighter patients [27].
A subsequent analysis from TRITON-TIMI 38 confirmed a consistent net clinical benefit of prasugrel from randomization to day 3, and from day 3 until the end of the trial [28]. Also, among patients who experienced a non-fatal event during the trial, there was a significant reduction in both recurrent events and subsequent CV death with prasugrel versus clopidogrel (HR 0.65, 95% CI 0.46–0.92, p = 0.016, and HR 0.46, 95% CI 0.25–0.82, p = 0.008, respectively) [29]. It should be noted that these are landmark analyses and further studies are needed to confirm these findings.
In contrast to TRITON-TIMI 38, the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY-ACS) trial failed to show superiority of prasugrel over clopidogrel (both on top of aspirin) in NSTE-ACS patients managed with medical therapy alone [30]. At 17 months, the composite rate of CV death, MI, and stroke with prasugrel treatment was 13.9 versus 16.0% with clopidogrel treatment (HR 0.91, 95% CI 0.79–1.05; p = 0.21). Although there were higher rates of minor and moderate bleeding among patients receiving prasugrel, there was no significant increase in the rate of severe, major, or life-threatening bleeding, despite a treatment duration up to 30 months. In this study, patients > 75 years or < 60 kg body weight received a reduced dose of prasugrel (5 mg rather than 10 mg); all patients received the same dose of clopidogrel (75 mg).
3.3.2 Ticagrelor
Ticagrelor is the first in a new class of agents called cyclopentyltriazolopyrimidines that reversibly inhibits the P2Y12 receptor by binding at a different site. It does not block ADP binding per se, but inhibits platelet activation by blocking ADP-induced signal transduction [5]. Unlike prasugrel, ticagrelor is a direct-acting agent with a faster onset of action than clopidogrel. Furthermore, it has a faster offset of action as a result of its reversible effects [16] (Table 1). It is recommended that ticagrelor is stopped at least 5 days prior to elective CABG surgery (class I recommendation), but shorter delays may be reasonable in patients referred for urgent CABG (class IIb recommendation) [9].
The pivotal ticagrelor trial was Platelet Inhibition and Patient Outcomes (PLATO), which evaluated the efficacy and safety of dual therapy with ticagrelor or clopidogrel plus aspirin for the reduction of CV events in patients hospitalized for either STEMI or moderate- to high-risk NSTE-ACS [25]. In contrast to TRITON-TIMI 38, patients were included whether or not an invasive strategy was planned. The study found that ticagrelor reduced the composite primary endpoint of CV death, MI, and stroke by 16% at 12 months compared with clopidogrel (HR 0.84, 95% CI 0.77–0.92; p < 0.001), but at the expense of an increase in the rate of PLATO- or TIMI-defined non–CABG-related major bleeding (TIMI-defined: 2.8 vs. 2.2%, p = 0.03) (Table 2). The individual endpoints of recurrent MI and CV death were also reduced in the ticagrelor group compared with clopidogrel (both p < 0.01) [25]. Moreover, ticagrelor treatment was associated with a significant reduction in the rate of death by any cause (4.5 vs. 5.9%; p < 0.001) [25], rates of both first and recurrent ischemic events [31], and rates of stent thrombosis (1.4 vs. 1.9%; p = 0.0091) [32]. A real-world evidence study conducted in Sweden (Swedish Web system for Enhancement and Development of Evidence-based care in Heart Disease Evaluated According to Recommended Therapies [SWEDEHEART]) and including over 45,000 ACS patients, subsequently reported outcomes for ticagrelor versus clopidogrel that were consistent with those found in PLATO [33].
Outcomes with ticagrelor versus clopidogrel in PLATO were consistent across subgroups of patients with STEMI [34] or NSTE-ACS [35], and those managed with either PCI [36] or medical therapy alone [37]. Similarly, outcomes were consistent in older patients, those with low body weight, and those with prior TIA or non-hemorrhagic stroke [25]. However, ticagrelor efficacy was found to differ according to region, with a reduced benefit in terms of the primary endpoint in patients based in North America compared with the rest of the world [25, 38]. As a greater proportion of patients in North America were reported to take high-dose aspirin maintenance therapy (median ≥ 300 mg/day), a negative interaction between ticagrelor and high-dose aspirin was proposed as a possible explanation for this disparity, but no definitive explanation exists for these findings [38]. As a result, ticagrelor maintenance therapy is recommended to be taken with low aspirin doses of 75–100 mg/day [9, 10]. The ticagrelor prescribing information also warns against concomitant aspirin doses exceeding 100 mg, and contraindicates the use of ticagrelor in patients with active pathological bleeding or history of intracranial hemorrhage [39].
3.3.3 Prasugrel Versus Ticagrelor
There are currently limited data comparing the efficacy and safety of ticagrelor and prasugrel in ACS patients. The results of the first head-to-head randomized clinical trial (PRimary Angioplasty in patients transferred from General community hospitals to specialized PTCA Units with or without Emergency thrombolysis-18 [PRAGUE-18]) were published recently [40, 41]. This open-label, phase IV study aimed to enroll 2500 patients with acute MI undergoing PCI in tertiary centers in the Czech Republic. However, early outcome analysis (up to 1 month post-event) of 1230 patients found no significant difference between prasugrel and ticagrelor (both plus aspirin) for the composite primary endpoint of death, re-infarction, urgent target vessel revascularization, stroke, serious bleeding requiring transfusion, or prolonging hospitalization at 7 days (4.0 and 4.1%, respectively; odds ratio [OR] 0.98, 95% CI 0.55–1.73; p = 0.939), nor in the key secondary endpoint of CV death, non-fatal MI, or stroke at 30 days (2.7 and 2.5%, respectively; OR 1.06, 95% CI 0.53–2.15; p = 0.864). Consequently, the trial was terminated early for ‘lack of utility’ [40]. The 1-year follow-up also found no significant differences between prasugrel and ticagrelor with regard to efficacy or bleeding. The primary endpoint (CV death, MI or stroke at 1 year) was 6.6% in the prasugrel group and 5.7% in the ticagrelor group (HR 1.167, 95% CI 0.742–1.835; p = 0.503). It should be noted that there are several limitations to this study, most notably that it was statistically underpowered to show superiority of one treatment over another. In addition, patients were allowed to switch to clopidogrel following discharge due to the high costs of prasugrel and ticagrelor in the Czech Republic. In fact, 34% of prasugrel patients and 44% of ticagrelor patients switched to clopidogrel for economic reasons; the mean time to switching was 8 days for both [41, 42].
An earlier meta-analysis of randomized trials of prasugrel and ticagrelor also showed no significant differences between treatments in the rates of CV death, MI or stroke, or non–CABG-related major bleeding [43]. This is an indirect comparative analysis. Recent pharmacodynamic studies suggest that there is little difference between prasugrel and ticagrelor in terms of timing and degree of platelet inhibition [44,45,46]. However, these studies have looked at only short-term pharmacodynamic effects after drug loading.
Other studies suggest that there may be a variable response with prasugrel when used long term or in patients with STEMI, influenced by older age and prior aspirin use [47, 48]. The open-label Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5 (ISAR-REACT 5) trial (NCT01944800) will compare the clinical effects of ticagrelor and prasugrel for up to 12 months in approximately 4000 ACS patients with a planned invasive strategy [49]. The estimated study completion date is January 2019.
In the absence of the contraindications referred to above, the most recent guidelines for maintenance treatment with dual antiplatelet therapy give a class IIa recommendation for the use of prasugrel or ticagrelor in preference to clopidogrel in ACS (NSTE-ACS or STEMI) patients who have undergone coronary stent implantation [50]. Ticagrelor (but not prasugrel) is recommended over clopidogrel in NSTE-ACS patients managed with medical therapy alone [50]. Other possible considerations in choice of agent include the dosing regimen and adverse event profile. Prasugrel is administered once daily and ticagrelor twice daily, which may have some bearing on patient compliance. In addition, ticagrelor is the only P2Y12 inhibitor that is currently licensed (according to prescribing information) to be crushed and mixed with water, and either drunk or given by nasogastric tube, for patients with difficulty swallowing [39]. Finally, both drugs carry an increased risk of bleeding (including life-threatening or fatal bleeding in the case of prasugrel), and ticagrelor is associated with an increased risk of dyspnea [27, 39].
3.4 Vorapaxar
Vorapaxar is a novel oral PAR-1 antagonist that inhibits thrombin-mediated platelet activation, which is independent of the ADP- and TXA-2-mediated pathways. Therefore, residual platelet activation is feasible despite dual inhibition of COX-1 and P2Y12, raising the question of whether ‘triple therapy’ would be beneficial.
The phase III study Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) investigated the efficacy and safety of vorapaxar versus placebo in NSTE-ACS patients receiving aspirin and clopidogrel, but was terminated early due to increased major bleeding with vorapaxar, including more than a three-fold increase in the rate of intracranial bleeding (HR 3.39, 95% CI 1.78–6.48, p < 0.05) [51]. There was also no apparent benefit of vorapaxar in reducing CV events.
Another study, the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events-Thrombolysis in Myocardial Infarction 50 (TRA 2°P-TIMI 50) study evaluated vorapaxar versus placebo in patients with a history of prior MI, stroke, or peripheral artery disease (PAD) (Table 2) [52]. At 3 years, although there was a significant reduction in the rate of CV death, MI, stroke, or recurrent ischemia leading to revascularization with vorapaxar versus placebo (11.2 vs. 12.4%; HR 0.88, 95% CI 0.82–0.95; p = 0.001), there was a significantly increased risk of moderate or severe bleeding with vorapaxar (4.2 vs. 2.5%; HR 1.66, 95% CI 1.43–1.93; p < 0.001), including intracranial hemorrhage (1.0 vs. 0.5%; p < 0.001). A subsequent subgroup analysis of patients with prior MI, and excluding all those with a high propensity to bleed (e.g., those with prior stroke or TIA, those aged over 75 years or weighing less than 60 kg), found that there was a greater reduction in the primary endpoint with vorapaxar (6.8 vs. 8.6%; HR 0.75, 95% CI 0.66–0.85; p < 0.0001) [53], but moderate or severe bleeding rates were still higher with vorapaxar compared with placebo (2.7 vs. 1.8%; HR 1.52, 95% CI 1.20–1.93; p = 0.0006), although they were lower than in the overall study. These results suggest the potential utility of vorapaxar in a selected population [53]. Indeed, vorapaxar has been approved by the US Food and Drug Administration (FDA) for secondary prevention in patients with prior MI or PAD, in combination with aspirin and/or clopidogrel, but is contraindicated in patients with a history of stroke, TIA or intracranial hemorrhage, or with active pathological bleeding [54]. The prescribing information also warns that consideration should be given to factors that increase the risk of bleeding, including older age and low body weight. The European guidelines recommend that ischemic and bleeding risk should be thoroughly assessed before prescribing vorapaxar with aspirin and clopidogrel [55]. However, the current US guidelines for the management of patients with NSTE-ACS and STEMI, and duration of dual antiplatelet therapy in coronary artery disease (CAD), do not refer to vorapaxar [9, 10, 50].
4 Optimal Duration of Treatment
4.1 Guidelines
Current US guidelines for ACS broadly recommend that dual antiplatelet therapy be continued for 12 months after the index event, followed by aspirin monotherapy [9, 10]. An American College of Cardiology (ACC)/American Heart Association (AHA) guideline focused update on the duration of dual antiplatelet therapy in patients with CAD was published recently, taking into account existing guideline recommendations and the results of a systematic review of randomized clinical trials [50, 56]. This update gives a class I recommendation for 12 months of treatment with low-dose aspirin (81 mg, range 75–100 mg) and a P2Y12 inhibitor in four specific groups of patients with an acute or recent coronary event (STEMI or NSTE-ACS), excluding those with specific contraindications to any of the drugs. These four ACS groups (with dual antiplatelet therapy recommendations) are (1) all medically managed patients (aspirin plus clopidogrel or ticagrelor); (2) STEMI patients treated with a fibrinolytic (aspirin plus clopidogrel); (3) patients who have undergone PCI with a drug-eluting stent (DES) or bare-metal stent (BMS) (clopidogrel, prasugrel, or ticagrelor); and (4) patients who have undergone CABG (resume treatment post-surgery and continue to 1 year). In the first three of these groups, the guidelines also give a class IIb recommendation that prolonging dual antiplatelet therapy beyond 12 months may be reasonable [50]. Conversely, dual antiplatelet therapy may be reasonable for just 6 months in patients with significant overt bleeding or at high bleeding risk (e.g., treatment with oral anticoagulant) or at increased risk of severe bleeding complication (e.g., major intracranial surgery). In patients with stable ischemic heart disease, the guidelines state that it may be reasonable to discontinue dual antiplatelet therapy sooner in PCI patients treated with ‘newer-generation’ DES (e.g., everolimus- or zotarolimus-eluting stents), as they are associated with a lower risk of stent thrombosis and MI compared with older DES types (e.g., sirolimus- and paclitaxel-eluting stents).
4.2 Evidence
Data from a number of clinical trials and recent meta-analyses indicating that extending dual antiplatelet therapy beyond 12 months may be beneficial in some patients are summarized below. Other studies have looked at shorter term dual antiplatelet therapy.
A subgroup analysis from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial in patients with prior MI found that ~ 2 years of treatment with clopidogrel plus aspirin reduced the rate of ischemic events by almost a quarter compared with aspirin therapy alone (6.6 vs. 8.3%; HR 0.77, 95% CI 0.6–0.98; p = 0.031) [57]; however, the trial failed to meet its primary endpoint, showing no benefit in patients with clinically evident CV disease or multiple risk factors [58].
The Dual Antiplatelet Therapy (DAPT) study showed that 30 months of treatment with clopidogrel or prasugrel plus aspirin reduced major CV event rates following coronary stent placement, compared with 12 months of treatment, although with an increased risk of bleeding and a suggestion of increased all-cause mortality (2.0 vs. 1.5%; HR 1.36, 95% CI 1.00–1.85; p = 0.05) [59]. Among a subgroup of patients undergoing PCI and stent placement following an MI in this study (30.7% of the randomized cohort), major CV and cerebrovascular event rates were significantly reduced in those who continued on a thienopyridine for 30 months versus those who switched to placebo at 12 months (3.9 vs. 6.8%; HR 0.56, 95% CI 0.42–0.76; p < 0.001) [60], but this was at the expense of a higher rate of Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Arteries (GUSTO) moderate or severe bleeding (1.9 vs. 0.8%, p = 0.005). There was no difference in all-cause mortality in this subgroup analysis.
In the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial, treatment with ticagrelor (90 or 60 mg twice daily) plus aspirin was extended over a 3-year period in stable patients with a history of MI (1–3 years prior to enrollment) [61]. Patients who received extended ticagrelor treatment experienced a significant reduction in the composite endpoint of CV death, MI, or stroke at 3 years (ticagrelor 90 mg 7.9%, ticagrelor 60 mg 7.8%, and placebo [aspirin alone] 9.0%; HR 0.85 for ticagrelor 90 mg vs. placebo 0.85, 95% CI 0.75–0.96, p = 0.008; and HR 0.84 for ticagrelor 60 mg vs. placebo, 95% CI 0.74–0.95, p = 0.004). The risk of major bleeding was higher with both doses of ticagrelor compared with placebo (ticagrelor 90 mg 2.6%, ticagrelor 60 mg 2.3%, and placebo 1.1%; p < 0.001 for each dose vs. placebo), but less than 1% of patients in each group experienced non-fatal intracranial hemorrhage or fatal bleeding over the 3-year period. The authors estimated that, for every 10,000 patients who began treatment with ticagrelor, 41 TIMI major bleeding events per year would be caused with 90 mg twice daily and 31 TIMI major bleeding events per year would be caused with 60 mg twice daily. It should be noted though that patients with known bleeding disorders, prior ischemic stroke or intracranial bleed, or those with a need for oral anticoagulant therapy were excluded from this study. As a result of this study, the recommended ticagrelor maintenance dose was updated to 90 mg twice daily for the first year post-ACS, and 60 mg twice daily thereafter [39]. The US FDA conducted their own analysis based on the PEGASUS-TIMI data to assess the benefit–risk difference of ticagrelor in a lower-risk population [62]. Their study found that ticagrelor compared with placebo consistently reduced the risk of MACE by approximately 16%, with no difference with respect to fatal bleeding or intracranial hemorrhages over 1 year.
In a meta-analysis of five randomized trials in high-risk patients with prior MI, extending P2Y12 inhibitor plus aspirin treatment beyond 1 year was shown to significantly decrease the risk of MACE (6.4 vs. 7.5%; risk ratio [RR] 0.78, 95% CI 0.67–0.90; p = 0.001) and CV death (2.3 vs. 2.6%; RR 0.85, 95% CI 0.74–0.98; p = 0.03) compared with aspirin alone [63]. Although there was an increase in major bleeding with extended dual antiplatelet therapy (1.85 vs. 1.09%; RR 1.73, 95% CI 1.19–2.50; p = 0.004), there was no significant increase in fatal bleeding or non-CV death. The authors of this meta-analysis did note that the studies they evaluated typically excluded patients with a high bleeding risk, such as those on long-term anticoagulant therapy, and/or with recent or active major bleeding, and history of prior stroke or TIA. Most of the patients were also biomarker-positive, indicating that they were at high risk of a recurrent event or CV death. As a result, the authors warned that the findings may not be generalizable to all ACS patients.
The duration of dual antiplatelet therapy following DES placement has been evaluated in a decision-analytic Markov model [64]. For the subgroup of patients with ACS, the authors found that only a 2% absolute reduction in MACE would be needed for 30 months of treatment with dual antiplatelet therapy to be preferable to 12 months followed by aspirin alone, including consideration of bleeding risk. However, a number of meta-analyses of randomized trials have generally shown that short-term (< 6 months) versus long-term (> 12 months) dual antiplatelet therapy after second-generation DES placement has similar rates of mortality and ischemic events, but with a lower rate of overall bleeding, particularly in low-risk patients [65,66,67,68,69]. The authors concluded that while shorter treatment may be safe and effective in some cases, high-risk patients may require a tailored approach. An analysis of 4190 patients from the Patterns of Non-Adherence to Anti-Platelet Regimen in Stented Patients (PARIS) registry found that only around 10% of patients treated with DES have either a low thrombotic/high bleeding risk or a high thrombotic/low bleeding risk [70]. Thus, identification of a high thrombosis/low bleeding risk or low thrombosis/high bleeding risk population is challenging.
A large, randomized, multicenter, open-label trial is currently assessing the hypothesis that 6 months of dual antiplatelet therapy after DES implantation is not inferior to 12 month dual antiplatelet therapy with regard to clinical outcomes. The final results of the study, known as the Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation in ST-elevation Myocardial Infarction (DAPT-STEMI), are awaited, but should hopefully help answer the question of whether short- or long-term dual antiplatelet therapy is preferential in patients with DES implantation [71].
5 Risk Stratification
In order to identify patients most likely to benefit from more intensive antiplatelet therapy, identification of characteristics associated with increased mortality, CV event recurrence, and bleeding is crucial. Bleeding risk is the primary safety issue associated with antiplatelet treatment and must be balanced against the reduction in ischemic risk when selecting therapy [50, 72]. Analysis of a prospective, real-world, Italian registry found that the main reason for continuing dual antiplatelet therapy beyond 12 months in patients following an ACS was low bleeding risk, more so than high ischemic risk [73]. Major bleeding events during hospitalization for ACS are an independent predictor of adverse outcomes at 6 months and 1 year post–index event [74,75,76]. An analysis of the PLATO trial found that spontaneous major bleeding events were associated with similar mortality rates (short and long term) as spontaneous ischemic events in patients with ACS receiving dual antiplatelet therapy [77]. A further study evaluated the average daily ischemic rate and the average daily bleeding rate in 3602 patients with STEMI enrolled in the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) study [78]. The study found that while both rates decreased over time after the primary PCI, the daily risk of ischemia was greater than the daily risk of bleeding after 30 days. To complicate matters, many factors that increase ischemic risk also increase the risk of bleeding [50] (Table 3).
Post-discharge risk scores currently include GRACE and the more recent risk model using data from the long-tErm follow-up of antithrombotic management Patterns In acute CORonary (EPICOR) study, which predict mortality at 6 months and 1 year following ACS, respectively [79, 80]. Most recently, a ‘DAPT score’ has been developed [81], using data from the DAPT study [59] to assess the potential benefits and harms of continuing dual antiplatelet therapy beyond 1 year in patients undergoing PCI [81]. This risk score has the advantage of evaluating both thrombotic and bleeding risk, with positive or negative points assigned for each of the components (Table 3). Patients with scores ≥ 2 were found to have a reduced risk of ischemic events and smaller increases in bleeding during extended dual antiplatelet therapy, compared with those with scores < 2 [81]. In another analysis looking at subgroups of patients with or without prior MI before coronary stent implantation, among patients with DAPT scores ≥ 2, continued thienopyridine therapy versus aspirin alone was associated with significant reductions in MI/stent thrombosis: prior MI 2.7 versus 6.0%, p < 0.001; no MI 2.6 versus 5.2%, p = 0.002, with comparable bleeding rates [82]. Among patients with DAPT scores < 2, continued thienopyridine therapy versus aspirin alone was associated with significantly increased bleeding, but no ischemic benefit, in patients with or without prior MI. Therefore, while the DAPT score may still require further evaluation in other patient cohorts, it has thus far been shown to enhance the prediction of relative benefit and harm with dual antiplatelet therapy. Alfredsson et al. utilized data from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes (TRILOGY ACS) study to identify predictors of long-term bleeding risks in patients with NSTEMI [83]. The authors identified ten significant predictors of GUSTO severe/life-threatening/moderate bleeding (age, sex, weight, NSTEMI [vs. unstable angina], angiography performed at randomization, prior peptic ulcer disease, baseline creatinine, baseline systolic blood pressure, baseline hemoglobin and angiography before randomization) and five significant predictors of TIMI major/minor bleed (age, female sex, baseline creatinine, baseline hemoglobin and angiography before randomization), which could be used to reliably predict bleeding risk in patients receiving dual antiplatelet therapy after hospitalization for ACS.
A number of individual patient factors are also recognized to increase the risk of CV events and/or bleeding, which may also have an impact on the relative benefits of dual antiplatelet therapy, as summarized briefly below. The risk of adverse events following an ACS also progressively increases with multiple risk factors [84]. For these patients, more aggressive secondary prevention strategies, such as longer dual antiplatelet therapy, may be required.
5.1 Diabetes Mellitus
Patients with diabetes mellitus have an increased risk of mortality and ischemic events, and a generally poorer prognosis following ACS, compared with non-diabetic patients [1, 85,86,87,88]. Patients receiving insulin therapy appear to be at further risk than those who do not require insulin [88]. Moreover, diabetic patients have been shown to have hyper-reactive platelets and reduced response to antiplatelet therapy compared with non-diabetic patients [89,90,91].
In PLATO, ticagrelor reduced the incidence of MACE in all ACS patients compared with clopidogrel, irrespective of diabetic status and glycemic control [25, 92]. In PEGASUS-TIMI 54, ticagrelor (pooled dose data) was again shown to reduce the relative risk of MACE consistently in both diabetic and non-diabetic patients over the 3-year follow-up period (p value for interaction = 0.99) [93]. However, because of the inherently greater degree of CV risk in diabetic patients, the absolute reduction in risk of ischemic events was greater in diabetic than non-diabetic patients (1.5 vs. 1.1%, with a 3-year number needed to treat of 67 vs. 91).
In TRITON-TIMI 38, the benefit of prasugrel in patients with diabetes compared with non-diabetic patients tended to be greater, with relative reductions in recurrent MI of 40% (8.2 vs. 13.2%; HR 0.60; p < 0.001) and 18% (7.2 vs. 8.7%; HR 0.82; p = 0.006), respectively (p value for interaction = 0.02) [94]. There was no significant interaction between treatment effect and diabetes status (p = 0.009) [26]. Rates of TIMI major bleeding were similar between patients with and without diabetes (p value for interaction = 0.29). It should be noted that this subset analysis was not powered to detect differences in individual endpoints.
In TRILOGY ACS, patients with diabetes, compared with those without diabetes, had a higher unadjusted and adjusted risk of all ischemic endpoints [88]. However, the frequencies of most ischemic and bleeding endpoints were similar for diabetics treated with prasugrel or clopidogrel (in combination with aspirin).
Intriguingly, a pre-specified subgroup analysis from the DAPT trial showed no benefit from extending dual antiplatelet therapy beyond 1 year in patients with diabetes [95]. The composite endpoint of death, MI or stroke occurred in 6.6% of diabetic patients with continued thienopyridine compared with 7.0% with placebo (p = 0.55); for non-diabetic patients, event rates were 3.3 versus 5.2%, respectively (p < 0.001). There was no mortality benefit with continued thienopyridine therapy versus placebo in either diabetic (2.7 vs. 2.2%, respectively; p = 0.32) or non-diabetic patients (1.5 vs. 1.2%; p = 0.28; p value for interaction = 0.96). Overall, the composite endpoint occurred more often in diabetic patients than in those without diabetes (6.8 vs. 4.3%, p < 0.001), as did the individual endpoints of death (2.5 vs. 1.4%, p < 0.001) and MI (4.2 vs. 2.6%, p < 0.001), although bleeding risk was similar regardless of diabetic status (p value for interaction = 0.61).
These findings suggest that caution needs to be exercised in deciding whether diabetic patients benefit more or less from dual antiplatelet therapy compared with non-diabetic patients. However, a recent meta-analysis showed that dual antiplatelet therapy is superior to aspirin alone in diabetic patients with ACS, and that both prasugrel and ticagrelor are better than clopidogrel in terms of CV event reduction, without an increased risk of major bleeding [96].
5.2 Renal Dysfunction
A significant proportion of patients with ACS have renal dysfunction, associated with poorer short- and long-term ischemic outcomes [97, 98]. However, renal dysfunction is associated with an increased risk of bleeding, complicating the net benefit–risk profile of potential antiplatelet therapy [50]. There is also evidence that a severe reduction in glomerular filtration rate may be a determinant of high residual platelet reactivity during clopidogrel maintenance therapy, and that the newer P2Y12 inhibitors may overcome this problem [99].
In a subgroup analysis of patients with CKD (creatinine clearance < 60 mL/min) included in the PLATO trial, ticagrelor significantly reduced the primary endpoint (composite of CV death, MI, and stroke) compared with clopidogrel (17.3 vs. 22.0%; HR 0.77, 95% CI 0.65–0.90) [100]. The absolute risk reduction was noted to be greater in patients with CKD than in those without it (7.9 vs. 8.9%; HR 0.90, 95% CI 0.79–1.02), with no differences in rates of major, fatal, or non–CABG-related bleeding. Similarly, in PEGASUS-TIMI 54, the relative reduction in CV events with ticagrelor and aspirin was similar among patients according to estimated glomerular filtration rate (eGFR), but absolute risk reduction was more marked in patients with eGFR < 60 mL/min/1.73 m2 (2.7 vs. 0.63%) due to a higher overall 3-year risk of CV events in this group [101].
In the TRITON-TIMI 38 trial, prasugrel was shown to be consistently superior to clopidogrel for reducing ischemic events in patients with or without CKD (creatinine clearance < 60 or ≥ 60 mL/min) [26]. In TRILOGY-ACS, however, while prasugrel treatment lowered platelet reactivity at 30 days across all patients regardless of kidney function, compared with clopidogrel, it did not affect either ischemic or bleeding outcomes in any group at 30 months [102].
The SWEDEHEART registry records baseline characteristics, treatments and outcome of consecutive patients with ACS admitted to all hospitals in Sweden. Patients prescribed dual antiplatelet therapy with clopidogrel and aspirin following an ACS were included in a prospective, observational cohort study to determine the optimal duration of treatment in patients with underlying renal disease [103]. Registered death, MI, stroke and bleeding events increased with worsening renal function, regardless of whether dual antiplatelet therapy was stopped at 3 months or continued beyond 3 months. The composite outcome of death, reinfarction, stroke or bleeding was in favor of longer dual antiplatelet therapy than shorter duration (HR 0.84, 95% CI 0.78–0.91).
The benefit of vorapaxar in patients with compromised kidney function is yet to be investigated on a large scale. Focused analyses evaluating the optimal antiplatelet regimen for patients with chronic renal dysfunction is a high priority.
5.3 Polyvascular Disease
Patients with vascular disease in more than one arterial bed are at a greater risk for ischemic events and have poorer prognosis following ACS [1, 57, 104].
Patients with polyvascular disease in CHARISMA had a marked reduction in MACE with clopidogrel and aspirin therapy versus aspirin alone (HR 0.55, 95% CI 0.33–0.91; p = 0.018) [57]. In PLATO, the benefit of ticagrelor in the subgroup of patients with ACS and PAD was consistent with the overall trial results, but did not reach statistical significance [104]. Similarly, outcomes from TRACER showed a trend toward a reduction in MACE in patients with NSTE-ACS and PAD with vorapaxar, compared with placebo, on background clopidogrel and aspirin, but the difference was not statistically significant [105]. As described above, patients with prior MI, stroke, or PAD in the TRA 2°P-TIMI 50 had a significant reduction in CV event rates with vorapaxar versus placebo, but with significantly increased bleeding risk [52]. Analysis from PEGASUS-TIMI 54 suggests that patients with prior MI and PAD have higher rates of MACE over a 3-year period and consequently experience a greater absolute risk reduction with ticagrelor and aspirin dual therapy versus aspirin alone, compared with patients without PAD, with an absolute excess of TIMI major bleeding of 0.12% (number needed to harm: 834) [106].
5.4 Age
Increasing age is associated with increased CV events and bleeding risk, reduced response to antiplatelet therapy, and a higher rate of HPR [107]. The net benefit of prasugrel was attenuated in patients > 75 years old in TRITON-TIMI 38 due to increased bleeding, and it is, therefore, not recommended for use in such patients [9, 10, 26]. In contrast, a PLATO substudy found that the overall trial results were consistent among patients < 75 or ≥ 75 years old [108]. Interestingly, PLATO-defined major bleeding with ticagrelor was not increased in older versus younger patients [50, 108]. Apart from these studies, data on the efficacy and safety of dual antiplatelet regimens in older patients are lacking. The ongoing POPular AGE study (NCT02317198) will compare clopidogrel with either ticagrelor or prasugrel (each on a background of aspirin or oral anticoagulant) in approximately 1000 NSTE-ACS patients ≥ 70 years old [109]. The study is due to be completed in January 2017.
6 Conclusion
The availability of new antiplatelet agents and extended or combination therapy has increased the options for secondary prevention among ACS patients. Clinicians should be guided by the results from the studies above and the subsequent clinical guidelines, but are reminded that individual patient circumstances and the benefit of antiplatelet treatment versus the risk of severe bleeding should be considered when deciding appropriate treatment.
Future trials should continue to highlight patient subgroups at high risk for recurrent ischemic events and at low risk for bleeding complications, who are likely to gain the greatest benefit from more potent and prolonged treatment.
References
Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304(12):1350–7.
Rapsomaniki E, Thuresson M, Yang E, Blin P, Hunt P, Chung S-C, et al. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors of myocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2016;2(3):172–83.
Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J. 2010;31(22):2755–64.
Alnasser SM, Huang W, Gore JM, Steg PG, Eagle KA, Anderson FA Jr, et al. Late consequences of acute coronary syndromes: global Registry of Acute Coronary Events (GRACE) follow-up. Am J Med. 2015;128(7):766–75.
Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12(1):30–47.
Stakos DA, Tziakas DN, Stellos K. Mechanisms of platelet activation in acute coronary syndromes. Curr Vasc Pharmacol. 2012;10(5):578–88.
Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86.
Jimenez AH, Stubbs ME, Tofler GH, Winther K, Williams GH, Muller JE. Rapidity and duration of platelet suppression by enteric-coated aspirin in healthy young men. Am J Cardiol. 1992;69(3):258–62.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362–425.
Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108(14):1682–7.
Berger JS, Sallum RH, Katona B, Maya J, Ranganathan G, Xu Y, et al. Is there an association between aspirin dosing and cardiac and bleeding events after treatment of acute coronary syndrome? A systematic review of the literature. Am Heart J. 2012;164(2):153–62.e5.
Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–43.
Grinstein J, Cannon CP. Aspirin resistance: current status and role of tailored therapy. Clin Cardiol. 2012;35(11):673–81.
Love BB, Biller J, Gent M. Adverse haematological effects of ticlopidine. Prevention, recognition and management. Drug Saf. 1998;19(2):89–98.
Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–85.
Bates ER, Lau WC, Angiolillo DJ. Clopidogrel-drug interactions. J Am Coll Cardiol. 2011;57(11):1251–63.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–20.
Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366(9497):1607–21.
Siller-Matula JM, Trenk D, Schror K, Gawaz M, Kristensen SD, Storey RF, et al. Response variability to P2Y12 receptor inhibitors: expectations and reality. JACC Cardiovasc Interv. 2013;6(11):1111–28.
Combescure C, Fontana P, Mallouk N, Berdague P, Labruyere C, Barazer I, et al. Clinical implications of clopidogrel non-response in cardiovascular patients: a systematic review and meta-analysis. J Thromb Haemost. 2010;8(5):923–33.
Cavallari LH, Beitelshees AL, Blake KV, Dressler LG, Duarte JD, Elsey A, et al. The IGNITE Pharmacogenetics Working Group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin Transl Sci. 2017;10(3):143–6.
Bristol-Myers Squibb/Sanofi Pharmaceuticals. PLAVIX (clopidogrel bisulfate) tablets. Prescribing Information. 2015. http://packageinserts.bms.com/pi/pi_plavix.pdf. Accessed 1 Sep 2017.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Eli Lilly. EFFIENT (prasugrel) tablets. Prescribing Information. 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022307s003lbl.pdf. Accessed 1 Sep 2017.
Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21):2028–33.
Murphy SA, Antman EM, Wiviott SD, Weerakkody G, Morocutti G, Huber K, et al. Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial. Eur Heart J. 2008;29(20):2473–9.
Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297–309.
Kohli P, Wallentin L, Reyes E, Horrow J, Husted S, Angiolillo DJ, et al. Reduction in first and recurrent cardiovascular events with ticagrelor compared with clopidogrel in the PLATO Study. Circulation. 2013;127(6):673–80.
Steg PG, Harrington RA, Emanuelsson H, Katus HA, Mahaffey KW, Meier B, et al. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation. 2013;128(10):1055–65.
Sahlén A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, et al. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37(44):3335–42.
Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131–41.
Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, Maya J, et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: results from the PLATO trial. Eur Heart J. 2014;35(31):2083–93.
Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283–93.
James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ. 2011;342:d3527.
Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124(5):544–54.
AstraZeneca. BRILINTA (ticagrelor) tablets. Prescribing Information. 2015. http://www.azpicentral.com/brilinta/brilinta.pdf. Accessed 1 Sep 2017.
Motovska Z, Hlinomaz O, Miklik R, Hromadka M, Varvarovsky I, Dusek J, et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: multicenter randomized PRAGUE-18 study. Circulation. 2016;134(21):1603–12.
Motovska Z, Hlinomaz O, Kala P, Hromadka M, Knot J, Varvarovsky I, et al. One-year outcomes of prasugrel versus ticagrelor in acute myocardial infarction treated with primary angioplasty: the PRAGUE-18 Study. J Am Coll Cardiol. 2018;71(4):371–81.
Bonaca MP, Wiviott SD. Prasugrel versus ticagrelor: uncertainty remains. Circulation. 2016;134(21):1613–6.
Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol. 2011;150(3):325–31.
Franchi F, Rollini F, Aggarwal N, Hu J, Kureti M, Durairaj A, et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 study. Circulation. 2016;134(11):780–92.
Bernlochner I, Mayer K, Orban M, Morath T, Jaitner J, Rossner L, et al. Ticagrelor versus prasugrel in patients with high on-clopidogrel treatment platelet reactivity after PCI: the ISAR-ADAPT-PF study. Platelets. 2016;27(8):796–804.
Rollini F, Franchi F, Cho JR, DeGroat C, Bhatti M, Muniz-Lozano A, et al. A head-to-head pharmacodynamic comparison of prasugrel vs. ticagrelor after switching from clopidogrel in patients with coronary artery disease: results of a prospective randomized study. Eur Heart J. 2016;37(35):2722–30.
Perl L, Zemer-Wassercug N, Rechavia E, Vaduganathan M, Orvin K, Weissler-Snir A, et al. Comparison of platelet inhibition by prasugrel versus ticagrelor over time in patients with acute myocardial infarction. J Thromb Thrombolysis. 2015;39(1):1–7.
Koifman E, Beigel R, Herscovici R, Fefer P, Rozenberg N, Sabbag A, et al. Immediate response to prasugrel loading in patients with ST-elevation myocardial infarction: predictors and outcome. Thromb Res. 2016;144:176–81.
Schulz S, Angiolillo DJ, Antoniucci D, Bernlochner I, Hamm C, Jaitner J, et al. Randomized comparison of ticagrelor versus prasugrel in patients with acute coronary syndrome and planned invasive strategy–design and rationale of the iNtracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial. J Cardiovasc Transl Res. 2014;7(1):91–100.
Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082–115.
Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366(1):20–33.
Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366(15):1404–13.
Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, et al. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2 degrees P-TIMI 50 trial. Lancet. 2012;380(9850):1317–24.
Merck & Co. Inc. Zontivity (vorapaxar) tablets. Prescribing Information. 2015. https://www.merck.com/product/usa/pi_circulars/z/zontivity/zontivity_pi.pdf. Accessed 1 Sep 2017.
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1116–39.
Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982–8.
Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66.
Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, et al. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65(20):2211–21.
Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800.
McDowell TY, Blank M, Lawrence J, Stockbridge N. Food and Drug Administration analysis of ticagrelor: using data from an enriched trial to evaluate benefit-risk difference in an unstudied population. Circulation. 2016;134(19):1500–2.
Udell JA, Bonaca MP, Collet JP, Lincoff AM, Kereiakes DJ, Costa F, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016;37(4):390–9.
Garg P, Galper BZ, Cohen DJ, Yeh RW, Mauri L. Balancing the risks of bleeding and stent thrombosis: a decision analytic model to compare durations of dual antiplatelet therapy after drug-eluting stents. Am Heart J. 2015;169(2):222–33.e5.
Bulluck H, Kwok CS, Ryding AD, Loke YK. Safety of short-term dual antiplatelet therapy after drug-eluting stents: an updated meta-analysis with direct and adjusted indirect comparison of randomized control trials. Int J Cardiol. 2015;181:331–9.
Palmerini T, Della Riva D, Benedetto U, Bacchi Reggiani L, Feres F, Abizaid A, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J. 2017;38(14):1034–43.
Bavishi C, Trivedi V, Singh M, Katz E, Messerli FH, Bangalore S. Duration of dual antiplatelet therapy in patients with an acute coronary syndrome undergoing percutaneous coronary intervention. Am J Med. 2017;130(11):1325.e1–.e12.
Basaraba JE, Barry AR. Short- versus standard-term dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent implantation: a meta-analysis. J Cardiol. 2017;69(1):353–8.
Wassef AW, Khafaji H, Syed I, Yan AT, Udell JA, Goodman SG, et al. Short duration vs standard duration of dual-antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents—a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. J Invasive Cardiol. 2016;28(12):e203–10.
Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, et al. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67(19):2224–34.
Kedhi E, Fabris E, van der Ent M, Kennedy MW, Buszman P, von Birgelen C, et al. A prospective, randomized, open-label trial of 6-month versus 12-month dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction: rationale and design of the “DAPT-STEMI trial”. Am Heart J. 2017;188:11–7.
Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32(15):1854–64.
Patti G, Cavallari I, Antonucci E, Calabro P, Cirillo P, Gresele P, et al. Prevalence and predictors of dual antiplatelet therapy prolongation beyond one year in patients with acute coronary syndrome. PLoS One. 2017;12(10):e0186961.
Rao SV, O’Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96(9):1200–6.
Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55(23):2556–66.
Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30(12):1457–66.
Ducrocq G, Schulte PJ, Budaj A, Cornel JH, Held C, Himmelmann A, et al. Balancing the risk of spontaneous ischemic and major bleeding events in acute coronary syndromes. Am Heart J. 2017;186:91–9.
Giustino G, Mehran R, Dangas GD, Kirtane AJ, Redfors B, Genereux P, et al. Characterization of the average daily ischemic and bleeding risk after primary PCI for STEMI. J Am Coll Cardiol. 2017;70(15):1846–57.
Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33.
Pocock S, Bueno H, Licour M, Medina J, Zhang L, Annemans L, et al. Predictors of one-year mortality at hospital discharge after acute coronary syndromes: a new risk score from the EPICOR (long-tErm follow uP of antithrombotic management patterns In acute CORonary syndrome patients) study. Eur Heart J Acute Cardiovasc Care. 2015;4(6):509–17.
Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735–49.
Kereiakes DJ, Yeh RW, Massaro JM, Cutlip DE, Steg PG, Wiviott SD, et al. DAPT score utility for risk prediction in patients with or without previous myocardial infarction. J Am Coll Cardiol. 2016;67(21):2492–502.
Alfredsson J, Neely B, Neely ML, Bhatt DL, Goodman SG, Tricoci P, et al. Predicting the risk of bleeding during dual antiplatelet therapy after acute coronary syndromes. Heart. 2017;103(15):1168–76.
Parodi G, Bellandi B, Tarantini G, Scudiero F, Valenti R, Marcucci R, et al. Clinical events beyond one year after an acute coronary syndrome: insights from the RECLOSE 2-ACS study. EuroIntervention. 2017;12(16):2018–24.
Cavender MA, Steg PG, Smith SC Jr, Eagle K, Ohman EM, Goto S, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) registry. Circulation. 2015;132(10):923–31.
Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–75.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34.
Dalby AJ, Gottlieb S, Cyr DD, Magnus Ohman E, McGuire DK, Ruzyllo W, et al. Dual antiplatelet therapy in patients with diabetes and acute coronary syndromes managed without revascularization. Am Heart J. 2017;188:156–66.
Amsallem M, Manzo-Silberman S, Dillinger JG, Sideris G, Voicu S, Bal dit Sollier C, et al. Predictors of high on-aspirin platelet reactivity in high-risk vascular patients treated with single or dual antiplatelet therapy. Am J Cardiol. 2015;115(9):1305–10.
Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64(10):1005–14.
Schuette C, Steffens D, Witkowski M, Stellbaum C, Bobbert P, Schultheiss HP, et al. The effect of clopidogrel on platelet activity in patients with and without type-2 diabetes mellitus: a comparative study. Cardiovasc Diabetol. 2015;14:15.
James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006–16.
Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF, et al. Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol. 2016;67(23):2732–40.
Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118(16):1626–36.
Meredith IT, Tanguay JF, Kereiakes DJ, Cutlip DE, Yeh RW, Garratt KN, et al. Diabetes mellitus and prevention of late myocardial infarction after coronary stenting in the randomized dual antiplatelet therapy study. Circulation. 2016;133(18):1772–82.
Rossington JA, Brown OI, Hoye A. Systematic review and meta-analysis of optimal P2Y12 blockade in dual antiplatelet therapy for patients with diabetes with acute coronary syndrome. Open Heart. 2016;3(1):e000296.
Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–65.
Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–95.
Morel O, Muller C, Jesel L, Moulin B, Hannedouche T. Impaired platelet P2Y12 inhibition by thienopyridines in chronic kidney disease: mechanisms, clinical relevance and pharmacological options. Nephrol Dial Transplant. 2013;28(8):1994–2002.
James S, Budaj A, Aylward P, Buck KK, Cannon CP, Cornel JH, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: results from the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2010;122(11):1056–67.
Magnani G, Storey RF, Steg G, Bhatt DL, Cohen M, Kuder J, et al. Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. Eur Heart J. 2016;37(4):400–8.
Melloni C, Cornel JH, Hafley G, Neely ML, Clemmensen P, Zamoryakhin D, et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: insights from the TRILOGY ACS Trial. Eur Heart J Acute Cardiovasc Care. 2016;5(6):443–54.
Carrero JJ, Varenhorst C, Jensevik K, Szummer K, Lagerqvist B, Evans M, et al. Long-term versus short-term dual antiplatelet therapy was similarly associated with a lower risk of death, stroke, or infarction in patients with acute coronary syndrome regardless of underlying kidney disease. Kidney Int. 2017;91(1):216–26.
Patel MR, Becker RC, Wojdyla DM, Emanuelsson H, Hiatt WR, Horrow J, et al. Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: data from the PLATO trial. Eur J Prev Cardiol. 2015;22(6):734–42.
Jones WS, Tricoci P, Huang Z, Moliterno DJ, Harrington RA, Sinnaeve PR, et al. Vorapaxar in patients with peripheral artery disease and acute coronary syndrome: insights from Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER). Am Heart J. 2014;168(4):588–96.
Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719–28.
Verdoia M, Pergolini P, Rolla R, Nardin M, Schaffer A, Barbieri L, et al. Advanced age and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. J Thromb Haemost. 2016;14(1):57–64.
Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, et al. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ Cardiovasc Qual Outcomes. 2012;5(5):680–8.
Qaderdan K, Ishak M, Heestermans AA, de Vrey E, Jukema JW, Voskuil M, et al. Ticagrelor or prasugrel versus clopidogrel in elderly patients with an acute coronary syndrome: optimization of antiplatelet treatment in patients 70 years and older–rationale and design of the POPular AGE study. Am Heart J. 2015;170(5):981–5.e1.
Moscucci M, Fox KAA, Cannon CP, Klein W, López-Sendón J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24(20):1815–23.
Morrow DA, Antman EM, Parsons L, de Lemos JA, Cannon CP, Giugliano RP, et al. Application of the TIMI risk score for ST-elevation MI in the National Registry of Myocardial Infarction 3. JAMA. 2001;286(11):1356–9.
Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119(14):1873–82.
Acknowledgments
Medical writing support was provided by Lucy Carter and Liz Anfield, and editorial support was provided by Nicola Jenkins, MA, both of Prime Global, Knutsford, Cheshire, UK, in accordance with Good Publication Practice guidelines (Link). Ultimate responsibility for opinions, conclusions, and data interpretation lies with the author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Medical writing support provided by Prime Global was funded by AstraZeneca. Open access fees were funded by AstraZeneca.
Conflict of interest
Dr. Berger has conducted research and sat on advisory boards for AstraZeneca and Janssen. He has also sat on an advisory board for Merck. Dr. Berger did not receive any financial incentive for preparing this manuscript and declares no other potential conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Berger, J.S. Oral Antiplatelet Therapy for Secondary Prevention of Acute Coronary Syndrome. Am J Cardiovasc Drugs 18, 457–472 (2018). https://doi.org/10.1007/s40256-018-0291-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-018-0291-2