Abstract
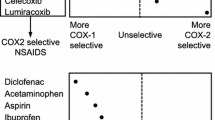
Non-steroidal anti-inflammatory drugs (NSAIDs) were differentiated from steroidal anti-inflammatory medicines to help clinicians who needed to use anti-inflammatory agents that were safer than steroids. With market entry of rofecoxib in 1999, NSAIDs were then further classified into traditional NSAIDs and cyclooxygenase (COX)-2 inhibitors (coxibs), the latter posing potentially fewer gastrointestinal risks. In 2005, rofecoxib was withdrawn from the market because of concerns about the risk of heart attack and stroke with long-term use, and clinical practice began focusing more on the cardiovascular versus gastrointestinal safety of coxibs. Since then, many coxibs have remained unapproved by the US FDA or have been removed from the market. This article explains how coxibs refocused attention on the cardiovascular safety of NSAIDs and the general implications of that. COX-2 activity/specificity is one factor associated with increased cardiovascular risks; however, these risks cannot be attributed to coxibs alone. The traditional NSAIDs (i.e., meloxicam, etodolac, and nabumetone) have significant COX-2 specificity, but naproxen and ibuprofen have less specificity. All NSAIDs, whether traditional or a coxib, pose some cardiovascular risks. It is possible that clinicians continue to focus more on decreasing the immediate gastric risks than preventing the later cardiovascular risks. The cardiovascular risks posed by NSAIDs should not be disregarded for the sake of achieving gastrointestinal benefits. Current recommendations suggest NSAIDs should be considered a single class of non-aspirin NSAIDs. Preferred NSAIDs are ibuprofen and naproxen. Coxibs are preferred in patients with low cardiovascular risk and high gastrointestinal risk who are intolerant to anti-dyspepsia therapy.
Similar content being viewed by others
References
Hawkey CJ, Skelly MM. Gastrointestinal safety of selective COX-2 inhibitors. Curr Pharm Des. 2002;8(12):1077–89.
Hawboldt J. Adverse Events Associated with NSAIDs. US Pharmacist. 2008;33(12):HS5-HS13. https://www.uspharmacist.com/article/adverse-events-associated-with-nsaids. Accessed 26 Jan 2017.
Sooriakumaran P. COX-2 inhibitors and the heart: are all coxibs the same? Postgrad Med J. 2006;82(966):242–5.
Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis—an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55.
Prakash S, Valentine V. Timeline: The Rise and Fall of Vioxx. NPR. http://www.npr.org/templates/story/story.php?storyId=5470430. Accessed 26 Sept 2016.
Borer JS, Simon LS. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: achieving a balance. Arthritis Res Ther. 2005;7(Suppl 4):S14–22.
Ong CKS, Lirk P, Tan CH, Seymour RA. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res. 2007;5(1):19–34.
Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events—is celecoxib the safest choice? Ther Clin Risk Manag. 2007;3(5):831–45.
NHS. Vioxx risk confirmed. NHS Choices. 2008. http://www.nhs.uk/news/2008/10October/Pages/Vioxxriskconfirmed.aspx. Accessed 26 Sept 2016.
Nissen SE, Yeomans ND, Solomon DH, et al. PRECISION Trial Investigators. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–29. doi:10.1056/NEJMoa1611593.
Cairns JA. The coxibs and traditional nonsteroidal anti-inflammatory drugs: a current perspective on cardiovascular risks. Can J Cardiol. 2007;23(2):125–31.
Loyd M, Rublee D, Jacobs P. An economic model of long-term use of celecoxib in patients with osteoarthritis. BMC Gastroenterol 2007;7:25. http://bmcgastroenterol.biomedcentral.com/articles/10.1186/1471-230X-7-25. Accessed 26 Sept 2016.
Rennie D. When evidence isn’t: Trials, drug companies and the FDA. J Law Policy. 2007:991–1012. http://brooklynworks.brooklaw.edu/cgi/viewcontent.cgi?article=1206&context=jlp. Accessed 26 Jan 2017.
US Food and Drug Administration. Safety: Bextra (valdecoxib) Apr 2005. Silver Spring (MD): US FDA; 2013. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150752.htm. Accessed 26 Sept 2016.
Geusens P, Lems W. Efficacy and tolerability of lumiracoxib, a highly selective cyclo-oxygenase-2 (COX2) inhibitor, in the management of pain and osteoarthritis. Ther Clin Risk Manag. 2008;4(2):337–44.
National University of Singapore, Faculty of Science. Relationship between structure, toxicity and activity. http://www.science.nus.edu.sg/research-highlights/1165-relationship-between-structure-toxicity-and-activity. Accessed 26 Sept 2016.
Government of Canada. Recalls and safety alerts. Withdrawal of Market Authorization for Prexige (lumiracoxib)—For Health Professionals. http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2007/14472a-eng.php. Accessed 26 Sept 2016.
Cannon CP, Curtis SP, FitzGerald GA, et al. MEDAL Steering Committee. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomized comparison. Lancet. 2006;368(9549):1771–81.
Drugs.com. Parecoxib approval status. https://www.drugs.com/history/parecoxib.html. Accessed 26 Sept 2016.
Drugs.com. Arcoxia approval status. https://www.drugs.com/history/arcoxia.html. Accessed 26 Sept 2016.
US Food and Drug Administration. FDA approves first generic versions of celecoxib [news release]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm399428.htm. Accessed 26 Sept 2016.
Solomon DH. Overview of selective COX-2 inhibitors. Uptodate.com. http://www.uptodate.com/contents/overview-of-selective-cox-2-inhibitors?source=see_link#H23. Accessed 26 Sept 2016.
Brooks MP. COX-2 inhibitors. Aust Prescr. 2000;23:30–2.
Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem. 2008;15(3):278–83.
Donati M, Conforti A, Lenti MC, et al. DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82(1):238–48. doi:10.1111/bcp.12938.
US Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. http://www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed 26 Sept 2016.
Harbin M, Turgeon RD, Kolber MR. Cardiovascular safety of NSAIDs. Can Fam Physician. 2014;60(3):e166.
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Jüni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. http://www.bmj.com/content/342/bmj.c7086. Accessed 26 Sept 2016.
Fraenkel L, Wittink DR, Concato J, Fried T. Informed choice and the widespread use of antiinflammatory drugs. Arthritis Rheum. 2004;51(2):210-4. http://onlinelibrary.wiley.com/doi/10.1002/art.20247/pdf. Accessed 4 Oct 2016.
Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15 Suppl 3:S2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3891482/. Accessed 4 Oct 2016.
Sequist TD. Patient preferences for treatment of Knee Osteoarthritis. Outcomes Res Rev. 2004;11(8):485-6. http://www.turner-white.com/memberfile.php?PubCode=jcom_aug04_knee.pdf. Accessed 4 Oct 2016.
Laba TL, Brien JA, Fransen M, Jan S. Patient preferences for adherence to treatment for osteoarthritis: the Medication Decisions in Osteoarthritis Study (MEDOS). BMC Musculoskelet Disord. 2013;14:160. http://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/1471-2474-14-160. Accessed 4 Oct 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Dixon Thomas, Zoya Ali, Seeba Zachariah, Kishore Gnana Sam Sundararaj, Matthew Van Cuyk, Jason C. Cooper declare that they have no conflict of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Thomas, D., Ali, Z., Zachariah, S. et al. Coxibs Refocus Attention on the Cardiovascular Risks of Non-Aspirin NSAIDs. Am J Cardiovasc Drugs 17, 343–346 (2017). https://doi.org/10.1007/s40256-017-0223-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-017-0223-6