Abstract
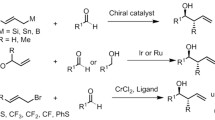
Allylation of bulky-substituted aromatic aldehydes with allyltrichlorosilanes were catalyzed by axial biscarboline N,N’-dioxide esters with high enantioselectivities up to 92% e.e. for 1-(4-chlorophenyl)-9-methyl-9H-pyrido[3,4-b]indole-3-carbaldehyde and 90% e.e. for 1-(3-methoxyphenyl)-9-methyl-9H-pyrido[3,4-b]indole-3-carbaldehyde, respectively. Total 22 aldehydes were tested with good yields and enantioselectivities. Catalyst 4f exhibited good catalytic enantioselectivity.
Similar content being viewed by others
References
Hosomi A., Sakurai H., Tetrahedron Lett., 1976, 16, 1295
Yus M., González-Gómez J. C., Foubelo F., Chem. Rev., 2011, 111, 7774
Cesarotti E., Araneo S., Rimoldi I., Tassi S., J. Mol. Catal. A: Chemical, 2003, 204/205, 221
Denmark S. E., Fu J. P., Chem. Rev., 2003, 103, 2763
Yus M., González-Gómez J. C., Foubelo F., Chem. Rev., 2013, 113, 5595
Denmark S. E., Beutner G. L., Wynn T., Eastgate M. D., J. Am. Chem. Soc., 2005, 127, 3774
Zheng K., Qin B., Liu X., Feng X. M., J. Org. Chem., 2007, 72, 8478
Malkov A. V., Kočovský P., Eur. J. Org. Chem., 2007, 2007, 29
Chelucci G., Murineddub G., Pinnab G. A., Tetrahedron: Asymmetry, 2004, 15, 1373
Nakajima M., Saito M., Uemura M., Hashimoto S., Tetrahedron Lett., 2002, 43, 8827
Nakajima M., Yokota T., Saito M., Hashimoto S., Tetrahedron Lett., 2004, 45, 61
Kina A., Shimada T., Hayash T. I., Adv. Synth. Catal., 2004, 346, 1169
Malkov A. V., Bell M., Orsini M., Pernazza D., Massa A., Herrmann P., Meghani P., Kočovský P., J. Org. Chem., 2003, 68, 9659
Malkov A. V., Dufková L., Farrugia L., Kočovský P., Angew. Chem., 2003, 115, 3802
Malkov A. V., Kysilka O., Edgar M., Kadlčíkov A., Kotora M., Kočovský P., Chem. Eur. J., 2011, 17, 7162
Denmark S. E., Fan Y., Tetrahedron: Asymmetry, 2006, 17, 687
Kadlčíkov A., Hrdina R., Valterová I., Kotora M., Adv. Synth.Catal., 2009, 351, 1279
Vlašan K., Hrdina R., Valterová I., Kotora M., Eur. J. Org. Chem., 2010, 7040
Jiao Z. G., Feng X. M., Liu B., Chen F. X., Zhang G. L., Jiang Y. Z., Eur. J. Org. Chem., 2003, 3818
Chelucci G., Belmonte N., Benagliab M., Pignataro L., Tetrahedron Lett., 2007, 48, 4037
Boyd D. R., Sharma N. D., Sbircea L., Murphy D., Malone J. F., James S. L. C. Allen C. R., Hamilton J. T. G., Org. Biomol. Chem., 2010, 8, 1081
Keck G. E., Tarbet K. H., Geraci L. S., J. Am. Chem. Soc., 1993, 115, 8467
Hanawa H., Hashimoto T., Maruoka K., J. Am. Chem. Soc., 2003, 125, 1708
Iseki K., Mizuno S., Kuroki Y., Kobayashi Y., Tetrahedron Lett., 1998, 39, 2767
Rauniyar V., Hall D. G., J. Org. Chem. 2009, 74, 4236
Bai B., Shen L., Ren J., Zhu H. J. Adv. Synth. Cat., 2012, 354, 354
Bruce D. R., [R-(R*R*)]-2-(4-Fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic Acid, Its Lactone form and Salts Thereof., US5273995, 1993
Sotiriou G. G., Cheng J. W., Ann. Parmacothor., 2009, 34, 1432
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.21877025), the Scientific Research Foundations of Hebei Educational Committee, China(Nos.QN2017021, ZD2017004) and the “Talent Program” of Hebei University, China.
Rights and permissions
About this article
Cite this article
Wang, J., Wu, S., Wang, X. et al. Enantioselective Addition of Allyltrichlorosilane to Bulky-substituted Aldehydes Catalyzed by Axial N,N′-Dioxide Pivalate. Chem. Res. Chin. Univ. 35, 604–608 (2019). https://doi.org/10.1007/s40242-019-9046-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-019-9046-0