Abstract
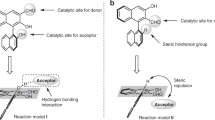
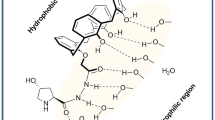
Chiral organocatalysts of 4-adamantane amide based on L-proline with double hydrogen potential were synthesized and used in asymmetric aldol reactions. The reactions were evaluated in toluene under‒20 °C. A series of aldol products was obtained from moderate to good yields(up to 98%) with excellent diastereoselectivities(up to >99:1) and enantioselectivities(up to >99%). The aldol products in the system were separated by α-cyclodextrin via host-guest interaction and determined by chiral HPLC. The catalyst could be reused up to five times. The 4-substitution position played an important role in diastereoselectivity and enantioselectivity.

Similar content being viewed by others
References
Sukumaran J., Hanefeld U., Chem. Soc. Rev., 2005, 34(6), 530
García-Urdiales E., Alfonso I., Gotor V., Chem. Rev., 2005, 105(1), 313
Sasaoka A., Uddin M. I., Shimomoto A., Ichikawa Y., Shiro M., Kotsukia H., Tetrahedron: Asymmetry, 2006, 17(21), 2963
Desimoni G., Faita G., Jørgensen K. A., Chem. Rev., 2006, 106(9), 3561
Mlynarski J., Paradowska J., Chem. Soc. Rev., 2008, 37(8), 1502
Naodovic M., Yamamoto H., Chem. Rev., 2008, 108(8), 3132
Bartók M., Chem. Rev., 2010, 110(3), 1663
Trost B. M., Brindle C. S., Chem. Soc. Rev., 2010, 39(5), 1600
Selander N. J., Szabó K., Chem. Rev., 2011, 111(3), 2048
Brovetto M., Gamenara D., Saenz M. P., Seoane G. A., Chem. Rev., 2011, 111(7), 4346
Mlynarski J., Baś S., Chem. Soc. Rev., 2014, 43(2), 577
Wang Y. C., Lin J., Wei K., Tetrahedron: Asymmetry, 2014, 25(24), 1599
List B., Lerner R. A., Barbas C. F., J. Am. Chem. Soc., 2000, 122(10), 2395
Huang X. R., Liu Q., Wang J., Xiao J. A., Yang H., Tetrahedron: Asymmetry, 2014, 25(24), 1590
Tang Z., Jiang F., Yu L., Cui X., Gong L., Mi A., Jiang Y., Wu Y., J. Am. Chem. Soc., 2003, 125(18), 5262
Tang Z., Jiang F., Cui X., Gong L. Z., Mi A. Q., Jiang Y. Z., Wu Y. D., Proc. Natl. Acad. Sci. U.S.A., 2004, 101, 5755
Tang Z., Yang Z. H., Chen X. H., Cun L. F., Mi A. Q., Jiang Y. Z., Gong L. Z., J. Am Chem. Soc., 2005, 127(25), 9285
Guizzetti S., Benaglia M., Pignataro L., Puglisi A., Tetrahedron: Asymmetry, 2006, 17(19), 2754
Tzeng Z., Chen H., Huang C., Chen K., Tetrahedron Lett., 2008, 49(26), 4134
Sato K., Kuriyama M., Shimazawa R., Morimoto T., Kakiuchi K., Shirai R., Tetrahedron Lett., 2008, 49(15), 2402
Chimi S. S., Singh S., Mahajan D., Tetrahedron: Asymmetry, 2008, 19(19), 2276
Zhang S. P., Fu X. K., Fu S. D., Pan J. F., Catal. Commun., 2009, 10(4), 401
Mitsui K., Hyatt S. A., Turner D. A., Hadad C. M., Parquette J. R., Chem. Commun., 2009, (22), 3261
Moorthy J. N., Saha S., Eur. J. Org. Chem., 2009, (5), 739
Saha S., Moorthy J. N., Tetrahedron Lett., 2010, 51(6), 912
Lu Z. J., Mei H. B., Han J. L., Pan Y., Chem. Biol. Drug Des., 2010, 76(2), 181
Kinsella M., Duggan P. G., Lennon C. M., Tetrahedron: Asymmetry, 2011, 22(13), 1423
Zhang F. R., Li C. M., Qi C. X., Tetrahedron: Asymmetry, 2013, 24(7), 380
Eymur S., Akceylan E., Sahin O., Uyanik A., Yilmaz M., Tetrahedron, 2014, 70(30), 4471
Akceylana E., Uyanika A., Eymurb S., Sahina O., Yilmaza M., Ak-ceylan E., Applied Catalysis A: General, 2015, 499, 205
Yadav G. D., Singh S., Tetrahedron: Asymmetry, 2016, 27, 123
Jin H., Cho S. M., Lee J., Ryu D. H., Org. Lett., 2017, 19(9), 2434
Izquierdo J., Pericàs M. A., ACS Catal., 2016, 6(1), 348
Benaglia M., Cinquini M., Cozzi F., Puglisi A., Celentano G., Adv. Synth. Catal., 2002, 344(5), 533
Benaglia M., Puglisi A., Cozzi F., Chem. Rev., 2003, 103(9), 3401
Cozzi F., Adv. Synth. Catal., 2006, 348(12/13), 1367
Gruttadauria M., Giacalone F., Marculescu A. M., Lo Meo P., Riela S., Noto R., Eur. J. Org. Chem., 2007, (28), 468
Lu A., Moatsuo D., Longbottom D. A., O’Reilly R. K., Chem. Sci., 2013, 4(3), 965
Xiao J., Li G. W., Zhang W., Chem. Res. Chinese Universities, 2013, 29(2), 256
Hu F. Y., Du G. H., Ye L., Zhu Y. T., Wang Y., Jiang L. M., Polymer, 2016, 102, 33
Font D., Bastero A., Sayalero S., Jimeno C., Pericas M. A., Org. Lett., 2007, 9(10), 1943
Alza E., Rodriguez-Escrich C., Sayalero S., Bastero A., Pericas M. A., Chem. Eur. J., 2009, 15(39), 10167
Riente P., Mendoza C., Pericas M. A., J. Mater. Chem., 2011, 21(20), 7350
Fan X., Alza E., Pericas M. A., RSC Adv., 2012, 2(14), 6164
Riente P., Yadav J., Pericas M. A., Org. Lett., 2012, 14(14), 3668
Caminade A. M., Ouali A., Keller M., Majoral J. P., Chem. Soc. Rev., 2012, 41(11), 4113
Rasmussen B., Christensen J. B., Org. Biomol. Chem., 2012, 10(25), 4821
Vaquer L., Riente P., Sala X., Jansat S., Benet-Buchholz J., Llobet A., Pericas M. A., Catal. Sci. Technol., 2013, 3(3), 706
Ribourdouille Y., Engel G. D., Gade L. H., Compets Rendus Chimie, 2003, 6(8―10), 1087
Slagt M. Q., Stiriba S. E., Kautz H., Gebbink R. J., Frey H., Koten G. V., Organometallics, 2004, 23(7), 1525
Itsuno S., Hassan M. M., RSC Adv., 2014, 4(94), 52023
Lu A., Cotanda P., Patterson J. P., Longbottom D. A., O’Reilly R. K., Chem. Commun., 2012, 48(78), 9699
Cooke G., Rotello V. M., Chem. Soc. Rev., 2002, 31(5), 275
Zhang Y., Wang W., Li Q., Yang Q. B., Li Y. X., Du J. S., Talanta, 2015, 141, 33
Cui B. Q., Yu J., Yu F. C., Li Y. M., Chang K. J., Shen Y. H., RSC Adv., 2015, 5(14), 10386
Huang W. P., Chen J. R., Li X. Y., Cao Y. J., Xiao W. J., Can. J. Chem., 2007, 85(3), 208
Zheng X., Qian Y. B., Wang Y. M., Eur. J. Org. Chem., 2010, 3, 515
Yarlagadda S., Ramesh B., Reddy C. R., Srinivas L., Sridhar B., Reddy B. V., Org. Lett., 2017, 19(1), 170
Uekama K. H., Irit T., Chem. Rev., 1998, 98, 2045
Sun T., Zhang H. C., Yan H., Li J. Y., Cheng G. H., Hao A. Y., Supramol. Chem., 2011, 23(5), 351
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.21174052) and the Natural Science Foundation of Jilin Province of China(Nos.20160101311JC, 20170101105JC).
Electronic supplementary material
40242_2018_7364_MOESM1_ESM.pdf
Role of adamantane amide at C4 based on L-proline double-H potential organocatalyst in aldol reaction with product being separated via host-gust interation
Rights and permissions
About this article
Cite this article
Wang, R., Wei, Z., Guo, J. et al. Role of Adamantane Amide Based on L-Proline Double-H Potential Organocatalyst in Aldol Reaction with Product Separated via Host-guest Interaction. Chem. Res. Chin. Univ. 34, 180–185 (2018). https://doi.org/10.1007/s40242-018-7364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-018-7364-2