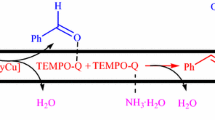
Abstract
A facile, practical and scalable catalyst system for alcohols ammoxidation into nitriles is developed using amino acid as ligand, oxygen as terminal oxidant and copper iodide(CuI) as catalyst. The catalyst system shows excellent functional groups compatibility for a wide range of testing substrates, even the substrates bearing oxidation-sensitive groups such as MeS—, alkenyl and —NH2 can also work well. In addition, the protocol is readily scaled up to more than 20 g and the product can be obtained just through filtration or distillation without conventional column chromatography.
Similar content being viewed by others
References
Hodgson H. H., Chem. Rev., 1947, 40(2), 251
Rappoport Z., The Chemistry of the Cyano Group, John Wiley & Sons, London, 1970
Fatiadi A. J., Preparation and Synthetic Applications of Cyano Compounds, Wiley, New York, 1983
Larock R. C., Comprehensive Organic Transformations: a Guide to Functional Group Preparations, John Wiley & Sons, New York, 1999
Fleming F. F., Nat. Prod. Rep., 1999, 16(5), 597
Liu K. C., Howe R. K., J. Org. Chem., 1983, 48(24), 4590
Harris T. M., Harris C. M. T., Oster A., Brown L. E., Lee J. Y. C., J. Am. Chem. Soc., 1988, 110(18), 6180
Galli C., Chem. Rev., 1988, 88(5), 765
Miller J. S., Manson J. L., Acc. Chem. Res., 2001, 34(7), 563
Martin A., Kalevaru V. N., Chem. Cat. Chem., 2010, 2(12), 1504
Yang C. H., Williams J. M., Org. Lett., 2004, 6(17), 2837
Cristau H. J., Ouali A., Spindler J. F., Taillefer M., Chem. Eur. J., 2005, 11(8), 2483
Mariampillai B., Alliot J., Li M. Z., Lautens M., J. Am. Chem. Soc., 2007, 129(49), 15372
Wang D. P., Kuang L. P., Li Z. W., Ding K., Synlett., 2008, (1), 69
Anbarasan P., Schareina T., Beller M., Chem. Soc. Rev., 2011, 40(10), 5049
Ushkov A. V., Grushin V. V., J. Am. Chem. Soc., 2011, 133(28), 10999
Yan G., Yu J., Zhang L., Chin. J. Org. Chem., 2012, 32(2), 294
Shim Y. J., Lee H. J., Park S., J. Organomet. Chem., 2012, 696(26), 4173
Magnus P., Scott D. A., Fielding M. R., Tetrahedron Lett., 2001, 42(25), 4127
Smith M. B., March J., March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; 6th Ed., Wiley, Hoboken, NJ, 2007
Wen Q. D., Jin J. S., Zhang L. P., Luo Y., Lu P., Wang Y. G., Tetrahedron Lett., 2014, 55(7), 1271
Fan Q. H., Ni N. T., Li Q., Zhang L. H., Ye X. S., Org. Lett., 2006, 6(5), 1007
Naoshi M., Hideo T., Synlett., 2005, 36(9), 1456
Iida S., Togo H., Tetrahedron, 2007, 63(34), 8274
Ren Y. M., Zhu Y. Z., Cai C., J. Chem. Res., 2008, (1), 18
Zhu C. J., Sun C. G., Wei Y. Y., Synthesis, 2010, (24), 4235
Hiroyuki S., Katsuhiko M., Hideo T., Synthesis, 2013, 45(15), 2155
Ishida T., Watanabeb H., Takei T., Hamasakia A., Tokunaga M., Haruta M., Appl. Catal. A: Gen., 2012, 425(3), 85
Reddy K. R., Maheswari C. U., Venkateshwar M., Prashanthi S., Kantam M. L., Tetrahedron Lett., 2009, 50(18), 2050
Shigekazu Y., Yasuyuki Y., Chem. Lett., 1990, (4), 571
Chen F. E., Li Y. Y., Jia H. Q., Synthesis, 2002, (13), 1804
Biondini D., Brinchi L., Germani R., Goracci L., Savelli G., Eur. J. Org. Chem., 2005, (14), 3060
Rokade B. V., Malekar S. K., Prabhu K. R., Chem. Commun., 2012, 48(44), 5506
Yadav D. K. T., Bhanage B. M., Eur. J. Org. Chem., 2013, 45(45), 5106
Tao C. Z., Liu F., Zhu Y. M., Liu W. W., Cao Z. L., Org. Biomol. Chem., 2013, 11(20), 3349
Jagadeesh R. V., Junge H., Beller M., Nature Commun., 2014, 5, 4123
Molla R. A., Ghosh K., Tuhina K., Islam S. M., New J. Chem., 2015, 39(2), 921
Oishi T., Yamaguchi K., Mizuno N., Angew. Chem. Int. Ed., 2009, 48(52), 6286
Tan D. W., Xie J. B., Li Q., Li H. X., Li J. C., Li H. Y., Lang J. P., Dalton Trans., 2014, 43(37), 14061
Xie J. B., Bao J. J., Li H. X., Tan D. W., Li H. Y., Lang J. P., RSC Adv., 2014, 4(96), 54007
Dornan L. M., Cao Q., Flanagan J. C. A., Crawford J. J., Cook M. J., Muldoon M. J., Chem. Commun., 2013, 49(54), 6030
Yin W. Y., Wang C. M., Huang Y., Org. Lett., 2013, 15(8), 1850
Hoover J. M., Stahl S. S., J. Am. Chem. Soc., 2011, 133(42), 16901
Hoover J. M., Ryland B. L., Stahl S. S., J. Am. Chem. Soc., 2013, 135(6), 2357
Hill N. J., Hoover J. M., Stahl S. S., J. Chem. Educ., 2013, 90(1), 102
Zhang G. F., Han X. W., Luan Y. X., Wang Y., Wen X., Ding C. R., Chem. Commun., 2013, 49(72), 7908
Zhang G. F., Lei J., Han X. W., Luan Y. X., Ding C. R., Shan S., Synlett., 2015, 26(6), 779
Saigo K., Kubota N., Takebayashi S., Hasegawa M., Bull. Chem. Soc. Jpn., 1986, 59(3), 931
Oishi T., Yamaguchi K., Mizuno N., Top. Catal., 2010, 53(7), 479
Nie R. F., Shi J. J., Xia S. X., Shen L., Chen P., Hou Z. Y., Xiao F. S., J. Mater. Chem., 2012, 22(35), 18115
Pérez V. T., Arriba A. F. D., Monleón L. M., Simón L., Rubio O. H., Sanz F., Morán J. R., Tetrahedron, 2014, 70(45), 8614
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.20702051) and the Natural Science Foundation of Zhejiang Province, China(No.LY13B020017).
Rights and permissions
About this article
Cite this article
Zhang, G., Zhang, G., Lei, J. et al. Aerobic alcohol ammoxidation catalyzed by copper(I)/amino acid: a scalable protocol to nitriles. Chem. Res. Chin. Univ. 32, 586–593 (2016). https://doi.org/10.1007/s40242-016-6067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-6067-9