Abstract
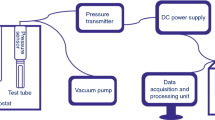
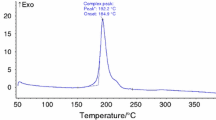
The thermal behavior and non-isothermal decomposition kinetics of [Cu(en)2H2O](FOX-7)2·H2O (en=ethylenediamine) were studied with DSC and TG-DTG methods. The kinetic equation of the exothermal process is dα/dt=(1017.92/β)4α 3/4exp(−1.688×105/RT). The self-accelerating decomposition temperature and critical temperature of the thermal explosion are 163.3 and 174.8 °C, respectively. The specific heat capacity of [Cu(en)2H2O](FOX-7)2·H2O was determined with a micro-DSC method, with a molar heat capacity of 661.6 J·mol−1·K−1 at 25 °C. Adiabatic time-to-explosion was also estimated as 23.2 s. [Cu(en)2H2O](FOX-7)2·H2O is less sensitive.
Similar content being viewed by others
References
Latypov N. V., Bergman J., Langlet A., Wellmar U., Bemm U., Tetrahedron, 1998, 54, 11525
Bemm U., Ötmark H., Acta Crystallogr. C, 1998, 54, 1997
Trzciński W. A., Cudzilo S., Chylek Z., Szymańczyk L., J. Hazard. Mater., 2006, 157, 605
Gao H. X., Zhao F. Q., Hu R. Z., Pan Q., Wang B. Z., Yang W. X., Gao Y., Gao S. L., Shi Q. Z., Chin. J. Chem., 2006, 24, 177
Zhao J. J., Liu H., Com. Mate. Sci., 2008, 42, 698
Huang B., Qiao Z. Q., Nie F. D., Cao M. H., Su J., Huang H., Hu C. W., J. Hazard. Mater., 2010, 184, 561
Venkatesan V., Polke B. G., Sikder A. K., Com. Theor. Chem., 2012, 995, 49
Rajappa S., Tetrahedron, 1981, 37, 1453
Hervé G., Jacob G., Latypov N., Tetrahedron, 2005, 61, 6743
Xu K. Z., Chang C. R., Song J. R., Zhao F. Q., Ma H. X., Lv X. Q., Hu R. Z., Chin. J. Chem., 2008, 26, 495
Luo J. A., Xu K. Z., Wang M., Song J. R., Ren Y. H., Chen Y. S., Zhao F. Q., Bull. Korean Chem. Soc., 2010, 31, 2867
Chen Y. S., Xu K. Z., Wang M., Ma H. X., Zhao F. Q., Chin. J. Explos. & Propellants, 2011, 34, 5
Garg S., Gao H. X., Parrish D. A., Shreeve J. M., Inorg. Chem., 2011, 50, 390
Vo T. T., Parrish D. A., Shreeve J. M., Inorg. Chem., 2012, 51, 1963
He F., Xu K. Z., Zhang H., Qiu Q. Q., Song J. R., Zhao F. Q., J. Coord. Chem., 2013, 66, 845
Gao Z., Huang J., Xu K. Z., Zhang W. T., Song J. R., Zhao F. Q., J. Coord. Chem., 2013, 66, 3572
Yang Q., Chen S. P., Xie G., Gao S. L., J. Hazard. Mater., 2011, 197, 199
Stierstorfer J., Tarantik K. R., Klapötke T. M., Chem. Eur. J., 2009, 15, 5775
Klapötke T. M., Stieratorfer J., Weber B., Inorg. Chim. Acta, 2009, 362, 2311
Kissinger H. E., Anal. Chem., 1957, 29, 1702
Ozawa T., Bull. Chem. Soc. Jpn., 1965, 38, 1881
Hu R. Z., Gao S. L., Zhao F. Q., Shi Q. Z., Zhang T. L., Zhang J. J., Thermal Analysis Kinetics, 2nd Ed., Science Press, Beijing, 2008
Vyzovkin S., Burnham A. K., Criado J. M., Maqueda L. A., Popescu C., Sbirrazzuoli N., Thermochim. Acta, 2011, 520, 1
Zhang T. L., Hu R. Z., Xie Y., Li F. P., Thermochim. Acta, 1994, 244, 171
Qiu Q. Q., Gao Z., Chen Y. S., Xu K. Z., Zhao F. Q., Chin. J. Energ. Mater., 2014, 22(2), 206
Xu K. Z., Song J. R., Zhao F. Q., Ma H. X., Gao H. X., Chang C. R., Ren Y. H., Hu R. Z., J. Hazard. Mater., 2008, 158, 333
Ma H. X., Yan B., Li Z. N., Guan Y. L., Song J. R., Xu K. Z., J. Hazard. Mater., 2009, 169, 1068
Xu K. Z., Chen Y. S., Wang M., Luo J. A., Song J. R., Zhao F. Q., Hu R. Z., J. Therm. Anal. Calorim., 2011, 105, 293
Lv L., Xu K. Z., Qiu Q. Q., Wang G., Song J. R., Zhao F. Q., Chem. Res. Chinese Uniersities, 2012, 28(5), 878
Tian Y. D., Zhao F. Q., Liu J. H., Handbook of Energetic Materials and the Related Compounds, National Defense Industry Press, Beijing, 2011
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(Nos. 21241003, 20803058), the Science and Technology Research and Development Program of Shaanxi Province, China(No.2013K02-25) and the Education Committee Foundation of Shaanxi Province, China(No.2013JK0697).
Rights and permissions
About this article
Cite this article
Zhang, Y., Dong, K., Qiu, Q. et al. Non-isothermal decomposition kinetics of [Cu(en)2H2O](FOX-7)2·H2O. Chem. Res. Chin. Univ. 30, 672–675 (2014). https://doi.org/10.1007/s40242-014-3549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-014-3549-5