Abstract
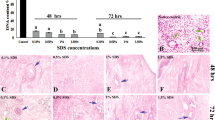
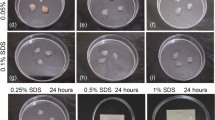
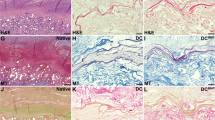
The present study was aimed to compare different decellularization protocols for human endometrial fragments. The freeze–thaw cycles in combination with treatment by Triton X-100 and four concentrations of sodium dodecyl sulfate (SDS; 0.1, 0.5, 1, and 1.5%) with two exposure times (24 and 72 h) were applied for tissues decellularization. After analysis the morphology and DNA content of tissues the group with better morphology and lower DNA content was selected for further assessments. The nucleus by Acridine orange and extracellular matrix (ECM) using Masson's trichrome, Alcian blue, and periodic acid–Schiff staining were studied. The amount of tissues collagen types I and IV, fibronectin, glycosaminoglycans (GAGs), and elastin was analyzed by Raman spectroscopy. The ultrastructure and porosity of decellularized scaffold were studied by scanning electron microscopy (SEM). The MTT assay was applied for assessments of cytotoxicity of scaffold. The treated group with 1% SDS for 72 h showed the morphology similar to native control in having the minimum level of DNA and well preserved ECM. Raman spectroscopy results demonstrated, the amount of collagen types I and IV, GAG, and fibronectin was not significantly different in decellularized scaffold compared with native group but the elastin protein level was significantly decreased (P < 0.001). SEM micrographs also showed a porous and fiber rich ECM in decellularized sample similar to the native control. This combined protocol for decellularization of human endometrial tissue is effective and it could be suitable for recellularization and clinical applications in the future.





Similar content being viewed by others
References
Aamodt JM, Grainger DW (2016) Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 86:68–82. https://doi.org/10.1016/j.biomaterials.2016.02.003
Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, Moffett A, Best S, Turco MY, Burton GJ, Cameron RE (2020) Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 10:20190079. https://doi.org/10.1098/rsfs.2019.0079
Agmon G, Christman KL (2016) Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr Opin Solid State Mater Sci 20:193–201. https://doi.org/10.1016/j.cossms.2016.02.001
Badylak SF (2007) The extracellular matrix as a biologic scaffold material. Biomaterials 28:3587–3593. https://doi.org/10.1016/j.biomaterials.2007.04.043
Baraga JJ, Feld MS, Rava RP (1992) In situ optical histochemistry of human artery using near infrared Fourier transform Raman spectroscopy. Proc NatL Acad Sci 89:3473–3477. https://doi.org/10.1073/pnas.89.8.3473
Campo H, Baptista PM, López-Pérez N, Faus A, Cervelló I, Simón C (2017) De-and recellularization of the pig uterus: a bioengineering pilot study. Biol Reprod 96:34–45. https://doi.org/10.1095/biolre/bio143396
Campo H, García-Domínguez X, López-Martínez S, Faus A, Antón JSV, Marco-Jiménez F, Cervelló I (2019) Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater 89:126–138. https://doi.org/10.1016/j.actbio.2019.03.004
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243. https://doi.org/10.1016/j.biomaterials.2011.01.057
Daryabari SS, Kajbafzadeh AM, Fendereski K, Ghorbani F, Dehnavi M, Rostami GBA, Tavangar SM (2019) Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J Assist Reprod Genet 36:1211–1223. https://doi.org/10.1007/s10815-019-01463-4
Das RS, Agrawal YK (2011) Raman spectroscopy: recent advancements, techniques and applications. Vib Spectrosc 57:163–176. https://doi.org/10.1016/j.vibspec.2011.08.003
Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, Grant SA (2011) Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater 96:199–206. https://doi.org/10.1002/jbm.b.31753
Dzobo K, Motaung KSCM, Adesida A (2019) Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 20:4628. https://doi.org/10.3390/ijms20184628
Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, D’Amore A, Badylak SF (2014) The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater 10:183–193. https://doi.org/10.1016/j.actbio.2013.09.006
Fayazi M, Salehnia M, Ziaei S (2017) In-vitro construction of endometrial-like epithelium using CD146+ mesenchymal cells derived from human endometrium. Reprod Biomed Online 35:241–252. https://doi.org/10.1016/j.rbmo.2017.05.020
Frushour BG, Koenig JL (1975) Raman scattering of collagen, gelatin, and elastin. Biopolymers 14:379–391. https://doi.org/10.1002/bip.1975.360140211
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–3683. https://doi.org/10.1016/j.biomaterials.2006.02.014
Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC (2014) Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant 33:298–308. https://doi.org/10.1016/j.healun.2013.10.030
Gratzer PF, Harrison RD, Woods T (2006) Matrix alteration and not residual sodium dodecyl sulfate cytotoxicity affects the cellular repopulation of a decellularized matrix. Tissue Eng 12:2975–2983. https://doi.org/10.1089/ten.2006.12.2975
Gu ZY, Jia SZ, Liu S, Leng JH (2020) Endometrial organoids: a new model for the research of endometrial-related diseases. BiolReprod 103:918–926. https://doi.org/10.1093/biolre/ioaa124
Han Q, Du Y (2020) Advances in the application of biomimetic endometrium interfaces for uterine bioengineering in female infertility. Front Bioeng Biotechnol 8:153. https://doi.org/10.3389/fbioe.2020.00153
He M, Callanan A, Lagaras K, Steele JAM, Stevens MM (2017) Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J Biomed Mater Res B Appl Biomater 105:1352–1360. https://doi.org/10.1002/jbm.b.33668
Hellström M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Bäckdahl H, Johansson BR, Olausson M, Sumitran-Holgersson S, Brännström M (2014) Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater 10:5034–5042. https://doi.org/10.1016/j.actbio.2014.08.018
Hellström M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K et al (2016) Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril 106:487–496. https://doi.org/10.1016/j.fertnstert.2016.03.048
Hiraoka T, Hirota Y, Saito-Fujita T, Matsuo M, Egashira M, Matsumoto L, Haraguchi H, Dey SK, Furukawa KS, Fujii T, Osuga Y (2016) STAT3 accelerates uterine epithelial regeneration in a mouse model of decellularized uterine matrix transplantation. JCI Insight 1:e87591. https://doi.org/10.1172/jci.insight.87591
Jakus AE, Laronda MM, Rashedi AS, Robinson CM, Lee C, Jordan SW, Orwig KE, Woodruff TK, Shah RN (2017) Tissue papers” from organ-specific decellularized extracellular matrices. Adv Funct Mater 27:1700992. https://doi.org/10.1002/adfm.201700992
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84:25–34. https://doi.org/10.1016/j.ymeth.2015.03.005
Kuo SY, Baker H, Fries MH, Yoo JJ, Kim PC, Fisher JP (2017) Bioengineering strategies to treat female infertility. Tissue Eng Part B Rev 23:294–306. https://doi.org/10.1089/ten.teb.2016.0385
Lim NSJ, Hamed Z, Yeow CH, Chan C, Huang Z (2011) Early detection of biomolecular changes in disrupted porcine cartilage using polarized Raman spectroscopy. J Biomed Opt 16:017003. https://doi.org/10.1117/1.3528006
Liu F, Hu S, Wang S, Cheng K (2019) Cell and biomaterial-based approaches to uterus regeneration. Regener Biomater 6:141–148. https://doi.org/10.1093/rb/rbz021
Matoba Y, Kisu I, Sera A, Yanokura M, Banno K, Aoki D (2019) Current status of uterine regenerative medicine for absolute uterine factor infertility. Biomed Rep 10:79–86. https://doi.org/10.3892/br.2019.1182
Mendibil U, Ruiz-Hernandez R, Retegi-Carrion S, Garcia-Urquia N, Olalde-Graells B, Abarrategi A (2020) Tissue-specific decellularization methods: rationale and strategies to achieve regenerative Compounds. Int J Mol Sci 21:5447. https://doi.org/10.3390/ijms21155447
Miki F, Maruyama T, Miyazaki K, Takao T, Yoshimasa Y, Katakura S, Hihara H, Uchida S, Masuda H, Uchida H, Nagai T, Shibata S, Tanaka M (2019) The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in rats. Biol Reprod 100:1215–1227. https://doi.org/10.1093/biolre/ioz004
Miyazaki K, Maruyama T (2014) Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 35:8791–8800. https://doi.org/10.1016/j.biomaterials.2014.06.052
Nguyen TT, Gobinet C, Feru J, Pasco SB, Manfait M, Piot O (2012) Characterization of type I and IV collagens by Raman microspectroscopy: Identification of spectral markers of the dermo-epidermal junction. J Spectrosc 27:421–427. https://doi.org/10.1155/2012/686183
Olalekan SA, Burdette JE, Getsios S, Woodruff TK, Kim JJ (2017) Development of a novel human recellularized endometrium that responds to a 28-day hormone treatment. Biol Reprod 96:971–981. https://doi.org/10.1093/biolre/iox039
Padma AM, Tiemann TT, Alshaikh AB, Akouri R, Song MJ, Hellström M (2018) Protocols for rat uterus isolation and decellularization: applications for uterus tissue engineering and 3D cell culturing. Methods Mol Biol 1577:161–175. https://doi.org/10.1007/7651_2017_60
Porzionato A, Stocco E, Barbon S, Grandi F, Macchi V, De Caro R (2018) Tissue-engineered grafts from human decellularized extracellular matrices: a systematic review and future perspectives. Int J Mol Sci 19:4117. https://doi.org/10.3390/ijms19124117
Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, Osuga Y, Saito S, Ushida T, Furukawa KS (2014) Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS ONE 9:e103201. https://doi.org/10.1371/journal.pone.0103201
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117. https://doi.org/10.1016/j.bbamem.2004.04.011
Strehle MA, Rösch P, Petry R, Hauck A, Thull R, Kiefer W, Popp J (2004) A Raman spectroscopic study of the adsorption of fibronectin and fibrinogen on titanium dioxide nanoparticles. Phys Chem Chem 6:5232–5236. https://doi.org/10.1039/B406524G
Tiemann TT, Padma AM, Sehic E, Bäckdahl H, Oltean M, Song MJ, Brännström M, Hellström M (2020) Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol Hum Reprod 26:167–178. https://doi.org/10.1093/molehr/gaaa009
Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ (2012) Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods 18:420–432. https://doi.org/10.1089/ten.tec.2011.0567
White LJ, Taylor AJ, Faulk DM, Keane TJ, Saldin LT, Reing JE, Swinehart IT, Turner NJ, Ratner BD, Badylak SF (2017) The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater 50:207–219. https://doi.org/10.1016/j.actbio.2016.12.033
Wu P, Nakamura N, Kimura T, Nam K, Fujisato T, Funamoto S, Higami T, Kishida A (2015) Decellularized porcine aortic intima-media as a potential cardiovascular biomaterial. Interact Cardiovasc Thorac Surg 21:189–194. https://doi.org/10.1093/icvts/ivv113
Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Neguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK (2017) A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8:1–3. https://doi.org/10.1038/ncomms14584
Xu H, Xu B, Yang Q, Li X, Ma X, Xia Q, Zhang Y, Zhang C, Wu Y, Zhang Y (2014) Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS ONE 9:e86723. https://doi.org/10.1371/journal.pone.0086723
Yao Q, Zheng YW, Lin HL, Lan QH, Huang ZW, Wang LF, Chen R, Xiao J, Kou L, Xu HL, Zhao YZ (2020) Exploiting crosslinked decellularized matrix to achieve uterus regeneration and construction. Artif Cells Nanomed Biotechnol 48:218–229. https://doi.org/10.1080/21691401.2019.1699828
Youngstrom DW, Barrett JG, Jose RR, Kaplan DL (2013) Functional characterization of detergent-decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS ONE 8:e64151. https://doi.org/10.1371/journal.pone.0064151
Acknowledgements
This work was supported by Tarbiat Modares University of Medical Sciences. The authors clarify that there is no conflict of interest. Special thanks of Mr. Pour Bayranvand for technical assistance.
Author information
Authors and Affiliations
Contributions
SM has supervised the research and prepared the manuscript, SZ has done the experiments, data analysis, and contributed to writing the paper, ZS was the scientific assistant of research and has done data analysis and revision of the manuscript, and JM has provided the human tissue samples.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sargazi, Z., Zavareh, S., Jafarabadi, M. et al. An efficient protocol for decellularization of the human endometrial fragments for clinical usage. Prog Biomater 10, 119–130 (2021). https://doi.org/10.1007/s40204-021-00156-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40204-021-00156-5