Abstract
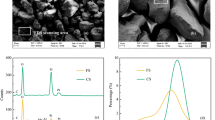
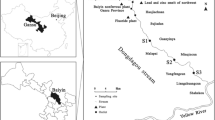
Adsorption of heavy metals on stream sediments has important implications for the fate and transport of contaminants in subsurface ecosystems. Lead (Pb) is a potentially hazardous heavy metal that is found in high amounts in anthropogenic environments, especially aquatic ecosystems. The key mechanisms for distributing this metal in the environment are adsorption and desorption in stream to sediment, and vice versa. Therefore, this work is mainly focused on the study of the influence of amorphous Fe/Al-oxyhydroxides and soil organic matter (SOM) on the adsorption of Pb onto natural stream sediment. Spiking adsorption experiments were carried out with four types of samples namely, untreated dried sediment, Fe/Al-oxyhydroxides depleted sediment, SOM depleted sediment and both Fe/Al as well as SOM depleted sediment in the pH range of 3.0 to 8.0. The results showed that Pb adsorption was reduced by up to 45% in amorphous Fe/Al-oxyhydroxide depleted sediment at pH 4.0 to 6.0, whereas a similar adsorption reduction was observed in SOM depleted sediment at pH 6.5 to 7.5. Maximum Pb adsorption was reduced by up to 75% in both amorphous Fe/Al-oxyhydroxides and SOM depleted sediment samples at pH ranges ranging from 3.0 to 7.0. Furthermore, it was shown that SOM was most significant at pH 6.5, while Fe/Al-oxyhydroxides were more important when pH was > 6.5 for the Pb adsorption in natural stream sediment.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [Pankaj Bakshe], upon reasonable request.
References
Panneerselvam B, Karuppannan S, Muniraj K. Evaluation of drinking and irrigation suitability of groundwater with special emphasizing the health risk posed by nitrate contamination using nitrate pollution index (NPI) and human health risk assessment (HHRA). Hum Ecol Risk Assess Int J. 2021;27:1324–48.
Belhouchette H, Boughariou E, Larayedh O, Bouri S. Groundwater quality evaluation and human health risks assessment using the WQI, NPI and HQnitrate models: case of the Sfax intermediate aquifer, Sahel Tunisia. Environ Geochem Health. 2022;44:2629–47.
Kılıç Z. The importance of water and conscious use of water. Int J Hydrol. 2020;4:239–41.
Li P, Karunanidhi D, Subramani T, Srinivasamoorthy K. Sources and consequences of Groundwater Contamination. Arch Environ Contam Toxicol. 2021;80:1–10.
Bakshe P, Jugade R, Distribution. Association, and ecological risk evaluation of heavy metals and influencing factors in major industrial stream sediments of Chandrapur District, Central India. Water Air Soil Pollut. 2021;232:78.
Rzetala M, Babicheva VA, Rzetala MA. Composition and physico-chemical properties of bottom sediments in the southern part of the Bratsk Reservoir (Russia). Sci Rep. 2019;9:12790.
Bradl HB. Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci. 2004;277:1–18.
Wang S, Mulligan CN. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J Hazard Mater. 2006;138:459–70.
Zhu J, Cozzolino V, Pigna M, Huang Q, Caporale AG, Violante A. Sorption of Cu, Pb and Cr on Na-montmorillonite: competition and effect of major elements. Chemosphere. 2011;84:484–9.
McKeague JA, Day JH. Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci. 1966;46:13–22.
Yin Y, Allen HE, Li Y, Huang CP, Sanders PF. Adsorption of mercury(II) by soil: effects of pH, chloride, and organic matter. J Environ Qual. 1996;25:837–44.
Agbenin JO, Olojo LA. Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma. 2004;119:85–95.
Pérez-Novo C, Pateiro-Moure M, Osorio F, Nóvoa-Muñoz JC, López-Periago E, Arias-Estévez M. Influence of organic matter removal on competitive and noncompetitive adsorption of copper and zinc in acid soils. J Colloid Interface Sci. 2008;322:33–40.
Shi Z, Allen HE, Di Toro DM, Lee S-Z, Harsh JB. Predicting PbII adsorption on soils: the roles of soil organic matter, cation competition and iron (hydr)oxides. Environ Chem. 2013;10:465.
Wang X, Liu F, Tan W, Li W, Feng X, Sparks DL. Characteristics of phosphate adsorption-desorption onto Ferrihydrite: comparison with Well-Crystalline Fe (Hydr)Oxides. Soil Sci. 2013;178:1–11.
Shuman LM. Adsorption of Zn by Fe and Al Hydrous Oxides as influenced by aging and pH1. Soil Sci Soc Am J. 1977;41:703.
Fisher-Power LM, Cheng T, Rastghalam ZS. Cu and Zn adsorption to a heterogeneous natural sediment: influence of leached cations and natural organic matter. Chemosphere. 2016;144:1973–9.
Mehra OP. Iron oxide removal from soils and clays by a Dithionite-Citrate System buffered with sodium bicarbonate. Clays Clay Miner. 1958;7:317–27.
Jackson ML. Soil chemical analysis. Englewood Cliffs: Prentice Hall Inc; 1958.
Watson ME, Brown JR. pH and lime requirement. Ecommended chem soil test proced North Cent Reg NCR Publ No 221. Columbia: Missouri Agricultural Experiment Station; p. 13–6.
Hendershot WH, Duquette M. A simple barium chloride method for determining Cation Exchange Capacity and Exchangeable cations. Soil Sci Soc Am J. 1986;50:605–8.
Jain CK. Metal fractionation study on bed sediments of River Yamuna, India. Water Res. 2004;38:569–78.
Boyer P, Wells C, Howard B. Extended K distributions for freshwater environment. J Environ Radioact. 2018;192:128–42.
Xu R, Zhao A, Yuan J, Jiang J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments. 2012;12:494–502.
Mizutani K, Fisher-Power LM, Shi Z, Cheng T. Cu and Zn adsorption to a terrestrial sediment: influence of solid-to-solution ratio. Chemosphere. 2017;175:341–9.
McBride M, Sauve S, Hendershot W. Solubility control of Cu, Zn, cd and pb in contaminated soils. Eur J Soil Sci. 1997;48:337–46.
Coston JA, Fuller CC, Davis JA. Pb2 + and Zn2 + adsorption by a natural aluminum- and iron-bearing surface coating on an aquifer sand. Geochim Cosmochim Acta. 1995;59:3535–47.
Acknowledgements
The authors are thankful to RTM Nagpur University for support under URPS, Dev-2117, DST, 584 New Delhi for DST-FIST grant and UGC, New Delhi for UGC-SAP grant.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pankaj Bakshe. The first draft of the manuscript was written by Pankaj Bakshe and conceptualization, writing: review and editing, supervision was done by Ravin Jugade. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bakshe, P., Jugade, R. Influence of Fe/Al oxyhydroxides and soil organic matter on the adsorption of Pb onto natural stream sediment. J Environ Health Sci Engineer (2024). https://doi.org/10.1007/s40201-024-00894-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40201-024-00894-1