Abstract
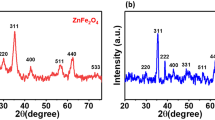
A novel approach has been adopted in the synthesis of nickel ferrite nanoparticles and their adsorption capacity was studied in the effective removal of MB dye from aqueous solution. Nanoparticles have a main advantage of treating large amount of wastewater within a short time and producing less contamination. The synthesized Spinel ferrites show high adsorption capacity, magnetic performance, and an eco-friendly material which effectively removes dyes. In the current work Nickel ferrite nanoparticles have been synthesized by wet hydroxyl chemical route using ethylene glycol as a chelating agent. XRD analysis indicates cubic spinel phase nickel ferrite and the average crystallite size is found to be 56.11 nm. An FTIR spectrum illustrates two intense absorption bands in the range between 1000 and 400 cm−1 corresponding to the presence of nickel ferrite. The shape and morphology of Nickel ferrite are examined by SEM analysis. The constituent elements and chemical composition analyzed using EDX spectrum showed that the estimated atomic percentages of O, Fe, and Ni are in good agreement with the theoretical value. VSM analysis clarifies soft ferromagnetic nature at room temperature. The equilibrium time for the removal of MB dye was found to be 180 mins. The capacity of nickel ferrite nanoparticles to adsorb the MB dye was proved from its maximum adsorption capacity of 72 mg g−1 from Langmuir model. The Equilibrium parameter (RL) and % error was calculated and found that Langmuir isotherm and Second-order kinetic model gave a good fit to the experimental data.










Similar content being viewed by others
References
Kadirvelu K, Kavipriya M, Karthika C, Radhika M, Vennilamani N, Pattabhi S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour Technol. 2003;87:129–32.
Patil MR, Shrivastava VS. Adsorptive removal of methylene blue from aqueous solution by polyaniline-nickel ferrite nanocomposite: a kinetic approach. Desalin Water Treat. 2016;57:5879–87.
El-Sharkawy EA, Soliman AY, Al-Amer KM. Comparative study for the removal of methylene blue via adsorption and photocatalytic degradation. J Colloid Interface Sci. 2007;310:498–508.
Sivakumar P, Ramesh R, Ramanand A, Ponnusamy S, Muthamizhchelvan C. Synthesis and characterization of NiFe2O4nanoparticles and nanorods. J Alloys Compd. 2013;563:6–11.
Verma A, Goel TC, Mendiratta RG, Gupta RG. High-resistivity nickel-zinc ferrites by the citrate precursor method. J Magn Magn Mater. 1999;192:271–6.
Caizer C, Stefanescu M. Magnetic characterization of nanocrystalline Ni-Zn ferrite powder prepared by the glyoxylate precursor method. J Phys D Appl Phys. 2002;35:3035–40.
Souza EA, Duque JGS, Kubota L, Meneses CT. Synthesis and characterization of NiO and NiFe2O4 nanoparticles obtained by a sucrose-based route. J Phys Chem Solids. 2007;68:594–9.
Baruwati B, Rana RK, Manorama SV. Further insights in the conductivity behavior of nanocrystalline Ni Fe2O4. J Appl Phys. 2007;101:014302.
Barati MR, Ebrahimi SAS, Badiei A. Influence of different calcination conditions on the microstructure and phase constitution of nickel-zinc ferrite nanocrystalline powders prepared by a sol-gel auto-combustion method. Key Eng Mater. 2008;372:598–600.
Zandipak R, Sobhanardakani S. Synthesis of NiFe2O4nanoparticles for removal of anionic dyes from aqueous solution. Desalin Water Treat. 2016;57:11348–60.
Afkhami A, Sayari S, Moosavi R, Madrakian T. Magnetic nickel zinc ferrite nanocomposite as an efficient adsorbent for the removal of organic dyes from aqueous solutions. The Korean Society of Industrial and Engineering Chemistry. J Ind Eng Chem. 2015;21:920–4.
Khosravi I, Eftekhar M. Characterization and evaluation catalytic efficiency of NiFe2O4nano spinel in removal of reactive dye from aqueous solution. Powder Technol. 2013;250:147–53.
Liu R, Fu H, Yin H, Wang P, Lu L, Tao Y. A facile sol combustion and calcination process for the preparation of magnetic Ni0.5Zn0.5Fe2O4nanopowders and their adsorption behaviors of Congo red. Powder Technol. 2015;274:418–25.
Zhang P, Lo I, O’Connor D, Pehkonen S, Cheng H, Hou D. High efficiency removal of methylene blue using SDS surface-modified ZnFe2O4nanoparticles. J Colloid Interface Sci. 2017;508:39–48.
Dehghani F, Hashemian S, Shibani A. Effect of calcination temperature for capability of MFe2O4 (M = Co, Ni and Zn) ferrite spinel for adsorption of bromophenol red. J Ind Eng Chem. 2017;48:36–42.
Zhao X, Wang W, Zhang Y, Wu S, Li F, Liu JP. Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for Congo Red. Chem Eng J. 2014;250:164–74.
Shirmardi M, Mahvi AH, Hashemzadeh B, Naeimabadi A, Hassani G, Niri MV. The adsorption of malachite green (MG) as a cationic dye onto functionalized multi walled carbon nanotubes. Korean J Chem Eng. 2013;30:1603–8.
Takdastan A, Mahvi AH, Lima EC, Shirmardi M, Babaei AA, Goudarzi G, et al. Preparation, characterization, and application of activated carbon from low-cost material for the adsorption of tetracycline antibiotic from aqueous solutions. Water Sci Technol. 2016;74:2349–63.
Shah I, Adnan R, Ngah WSW, Mohamed N. Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: regeneration and kinetics studies. PLoS One. 2015;10:1–23.
Boobalan T, Pavithradevi S, Suriyanarayanan N, Raja MM, Kumar ER. Preparation and characterization of polyol assisted ultrafine Cu–Ni–Mg–Ca mixed ferrite via co-precipitation method. J Magn Magn Mater. 2017;428:382–9.
Maaz K, Karim S, Mumtaz A, Hasanain SK, Liu J, Duan JL. Synthesis and magnetic characterization of nickel ferrite nanoparticles prepared by co-precipitation route. J Magn Magn Mater. 2009;321:1838–42.
Khot SS, Shinde NS, Ladgaonkar BP, Kale BB, Watawe SC. Magnetic and structural properties of magnesium zinc ferrites synthesized at different temperature. Adv Appl Sci Res. 2011;2:460–71.
Chakraverty S, Bandyopadhyay M. Coercivity of magnetic nanoparticles: a stochastic model. J Phys Condens Matter. 2007;19:216201.
Kahn ML, Zhang ZJ, Kahn ML, Zhang ZJ. Synthesis and magnetic properties of CoFe2O4 spinel ferrite nanoparticles doped with lanthanide ions synthesis and magnetic properties of CoFe2O4 spinel ferrite nanoparticles doped with lanthanide ions. Appl Phys Lett. 2001;3651:4–7.
Cazetta AL, Vargas AMM, Nogami EM, Kunita MH, Guilherme MR, Martins AC, et al. NaOH-activated carbon of high surface area produced from coconut shell: kinetics and equilibrium studies from the methylene blue adsorption. Chem Eng J. 2011;174:117–25.
Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–403.
Ebrahimian Pirbazari A, Saberikhah E, Habibzadeh Kozani SS. Fe3O4-wheat straw: preparation, characterization and its application for methylene blue adsorption. Water Resour Ind. 2014;7–8:23–37.
Vîrlan C, Ciocârlan RG, Roman T, Gherca D, Cornei N, Pui A. Studies on adsorption capacity of cationic dyes on several magnetic nanoparticles. Acta Chemica Iasi. 2013;21:19–30.
Hameed BH, Ahmad AL, Latiff KNA. Adsorption of basic dye (methylene blue) onto activated carbon prepared from rattan sawdust. Dyes Pigments. 2007;75:143–9.
Ashrafi SD, Kamani H, Soheil Arezomand H, Yousefi N, Mahvi AH. Optimization and modeling of process variables for adsorption of basic blue 41 on NaOH-modified rice husk using response surface methodology. Desalin Water Treat. 2016;57:14051–9.
Ashrafi SD, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA. The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, Paraconiothyrium variabile. Int Biodeterior Biodegrad. 2013;85:173–81.
Mahmoodi NM. Nickel ferrite nanoparticle: synthesis, modification by surfactant and dye removal ability. Water Air Soil Pollut. 2013;224:1–11.
Ho YS, McKay G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng. 1998;76:822–7.
Ahmaruzzaman M, Gayatri SL. Activated tea waste as a potential low-cost adsorbent for the removal of p -nitrophenol from wastewater. J Chem Eng Data. 2010;55:4614–23.
Hou X, Feng J, Liu X, Ren Y, Fan Z, Zhang M. Magnetic and high rate adsorption properties of porous Mn1-xZnxFe2O4 (0≤x≤0.8) adsorbents. J Colloid Interface Sci. 2011;353:524–9.
Acknowledgments
The authors are grateful for the financial support given by the Management of MepcoSchlenk Engineering College. The authors are also thankful to Thiru. A. Tenzing, Correspondent, and Dr. S. Arivazhagan, Principal, and Dr. A. Marikani, Senior Professor and Head, Department of Physics, MepcoSchlenk Engineering College, Sivakasi, for their constant support and encouragement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There is no conflict of interest in the submission.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
B, G.M., J B, M., P, R. et al. Equilibrium and kinetic studies on methylene blue adsorption by simple polyol assisted wet hydroxyl route of NiFe2O4nanoparticles. J Environ Health Sci Engineer 17, 539–547 (2019). https://doi.org/10.1007/s40201-019-00368-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-019-00368-9