Abstract
Purpose
This observational trial was performed to evaluate liver parameters in overweight or obese subjects in the context of insulin resistance and glucose control over time.
Subjects/Methods
Insulin resistance, glucose control and several parameters for liver integrity were monitored in 177 overweight (BMI > 28 kg/m2) subjects over a mean of 30 months. Volunteers were categorized according to insulin resistance (HOMAIR score) and glucose control in subjects with normal glucose control (NGT), impaired glucose control (IGT), or diabetes mellitus type 2 (T2DM). Liver fat and fibrosis were evaluated by sonographic elastography (FibroScan®) and clinical scores, such as the AST/ALT ratio, fatty liver index (FLI), and NAFLD fibrosis score (NFS).
Results
Liver fat fraction as estimated by the controlled attenuation parameter (CAP), and the FLI were significantly higher in subjects with T2DM compared to IGT and NGT. While fasting insulin levels and the HOMAIR score continuously increased over time, no change in CAP or FLI occurred during follow up. CAP was correlated with FLI (r = 0.50; p < 0.0001) and the HOMAIR score (r = 0.32; p < 0.0001). An inverse correlation was observed between serum adiponectin levels and FLI (r = -0.37; p < 0.0001), the HOMAIR score (r = -0.19; p < 0.001, and CAP (r = -0.15; p < 0.01).
Conclusions
In subjects with a BMI ≥ 28 kg/m2, liver fat fraction is significantly elevated in those with T2DM compared to IGT or NGT. Liver fat fraction is associated with deteriorating insulin sensitivity and loss of glucose control. Despite a continuous increase in insulin resistance, no change in liver fat content or stiffness occurred over 30 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has emerged as one of the most prominent metabolic disorders with serious consequences for health care systems worldwide. Obesity is most often accompanied by several health-threatening conditions, such as fatty liver disease, insulin resistance, systemic inflammation, lipid disorders and deteriorating glucose control. An increase in adipose tissue and the development of insulin resistance with a high flow of free fatty acids from adipocytes to the liver is a central mechanism in the development of fatty liver disease and liver fibrosis [1, 2]. Since treatment and prognosis of infective hepatitis have markedly improved in recent years, NAFLD has become the most common cause of severe liver injury, with a high risk of progressing to steatohepatitis, liver cirrhosis, or hepatocellular carcinoma [3, 4]. Beyond these hepatic complications, NAFLD markedly increases the risk of cardiovascular complications, heart failure, and chronic kidney disease [5,6,7,8,9]. Insulin resistance is hypothesized to be the main pathophysiological link between NAFLD and these non-hepatic complications [10]. Currently, medical societies realize that NAFLD is a significant health care challenge with a high need for early medical attention.
The results presented here are generated from a longitudinal observational trial tracking several biomarkers and clinical and sonographic parameters for insulin resistance and glucose control over a period of 30 months in overweight or obese subjects. The study design, methods and baseline data of the study participants have been published in detail previously [11].
Materials and methods
Patients and study design
The data presented here are retrieved from two follow-up visits of a longitudinal survey in overweight or obese subjects. The study was performed in accordance with the Declaration of Helsinki. The study was approved by the local ethical review board and was registered in the German registry of clinical studies (registration number DRKS00017516).
Criteria for inclusion in the study were a BMI greater than 28 kg/m2, male or female subjects between 18 and 80 years, subjects to be able to understand and follow the study instructions, and a signed informed consent. Subjects were excluded if they had diabetes mellitus type 1, maltose malabsorption, acute gastrointestinal disorders, systemic treatment with corticoids, pregnant or nursing women, active implantable medical devices (e.g., pacemaker), ascites, acute hepatitis, positive results in hepatitis or human immunodeficiency test, or a history of alcohol abuse.
Subjects were categorized as patients with diabetes mellitus type (T2DM) according to their medical history based on increased fasting glucose levels, HbA1c levels, or an oral glucose test. Patients with T2DM were included in the study independent from their glucose lowering treatments (lifestyle, metformin, sulfonylurea, DPP-IV inhibitors, sGLT-2 inhibitors, GLP-1 receptor agonists, or insulin. Participants without a history of diabetes mellitus type 2 (T2DM) underwent a standardized oral glucose tolerance test containing 75 g glucose solved in 300 ml water at all three visits. Subjects were allocated to the IGT group if their blood glucose concentration at 120 min after glucose intake was between 140 and 199 mg/dL (7.8 and 11.0 mmol/L). Subjects with a glucose level greater than 199 mg/dL two hours after glucose intake were allocated to the T2DM group.
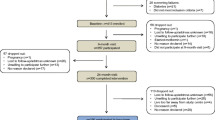
At the baseline visit (V1) and at two follow-up visits after approximately 15 (V2) and 30 months (V3), the study participants entered the study sites in the morning after fasting overnight for at least 10 h. Fasting blood samples were taken for the measurement of glucose, insulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (gamma-GT), triglycerides, thrombocytes, serum albumin, leptin and adiponectin levels.
Insulin, AST, ALT, gamma-GT, triglycerides, and albumin were measured in serum. Fasting glucose was measured in sodium fluoride (NaF) plasma using Cobas® 6000 analysers (Roche, Germany). Thrombocytes were analysed in EDTA blood using a Sysmex XN-1000 blood counter (Sysmex Deutschland GmbH, Germany). Leptin was analysed in serum with an ELISA from DRG Instruments. Total adiponectin was analysed in serum with an ELISA from R&D Systems.
Clinical and biomarker scores
HOMAIR score
The HOMAIR score was used to estimate insulin resistance [12]. The HOMAIR score was calculated from fasting glucose and insulin concentrations as follows:
Fatty liver index (FLI)
FLI is an established measure for a non-invasive estimate of liver fat content [13]. The FLI value is calculated based on the laboratory markers triglycerides and gamma-GT and the body composition markers BMI and waist circumference according to the formula:
where e is Euler’s number 2.71828 and y is 0.953 × ln (triglycerides, mg/dL) + 0.139 × BMI, kg/m2 + 0.718 × ln (gamma-GT, U/L) + 0.053 × waist circumference, cm – 15.745) [14].
NAFLD fibrosis score (NFS)
NFS is a validated score for estimating the severity of liver fibrosis.
This composite score is calculated based on the factors age, hyperglycaemia (i.e., impaired fasting glucose [IFG]/diabetes), BMI, platelet count (thrombocytes), AST/ALT ratio (AAR), and albumin as follows:
Liver fat and stiffness
Liver fat and stiffness were measured using transient elastography (FibroScan®, Echosens, Paris, France). The FibroScan® device evaluates liver fat content given by the controlled attenuation parameter (CAP), expressed as decibels per metre (dB/m), as well as liver stiffness given as the Young’s modulus (E), expressed as kilopascal (kPA) [15, 16].
Statistical analysis
For statistical comparisons between study groups and other variables of interest (e.g., HOMAIR and fibrosis categories), the type of analysis was chosen based on the type of data (numerical/categorical) and number of groups compared.
If a numerical variable was compared among more than two groups, 95% confidence intervals of the numerical variables for each group were added to the summary statistics. The overall influence of the grouping variable was assessed by the p value of the F test of a simple analysis of variance (ANOVA) model using the grouping variable as the single independent variable. Pairwise differences were assessed by calculating the least square mean difference and the 95% confidence interval. The statistical significance of differences was assessed using the p value of the corresponding t test.
If a numerical variable was compared between two groups, 95% confidence intervals of the numerical variables by group were added to the summary statistics. Groups were compared by calculating the arithmetic mean difference, parametric 95% confidence interval, and p value of the corresponding t test.
If a binary variable was compared among two or more groups, groups were compared overall and pairwise using a chi-square test.
Furthermore, linear regressions were performed using liver stiffness and liver fat as dependent variables and HOMAIR, AST/ALT ratio, FLI and NFS as independent variables. Pearson correlation coefficients were calculated for all variables with a p value < 0.05 in the regression analyses.
For group comparisons, raw p values are given. A p value < 0.05 was considered statistically significant.
Results
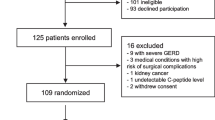
At baseline (V1), 301 overweight subjects with a BMI > 28 kg/m2 were enrolled in the study and were categorized according to their insulin sensitivity and diabetic status [11]. After a mean of 15 months (V2) 240, and after a mean of 30 months (V3) 177 subjects were available for follow up. The data presented here rely on the subset of 177 subjects who could be followed for the whole duration of 871 ± 142 days (mean ± SD). Of these 177 subjects (72 female, 105 male), 88 were with NGT, 26 were with IGT, and 63 subjects appeared with T2DM. Over the entire observational period, 18 out of 88 subjects (20%) deteriorated from NGT to IGT, and 2 out of 26 (8%) deteriorated from IGT to T2DM.
As shown in Table 1, the mean body weight over time slightly increased in the NGT group, remained unchanged in the IGT group, and slightly declined in the T2DM group. BMI increased by 0.49 ± 1.9 kg/m2 (mean ± SD; p < 0.05) in the NGT group and declined by 0.6 ± 1.8 kg/m2 (p < 0.05) in the T2DM group. Overall fasting insulin levels increased by 1.7 ± 9.9 mU/L at V2 and by 2.33 ± 8.6 mU/l at V3 (p < 0.001). This was mainly driven by an increase in insulin levels in the NGT group by 1.9 ± 5.7 (p < 0.01) and by 2.9 ± 11.9 mU/L (p = 0.06) in the T2DM group. Insulin resistance as evaluated with the HOMAIR score was significantly higher in the T2DM group than in the IGT and NGT groups (Table 1). In all subjects, the HOMAIR score increased by 0.5 ± 3.1 (p < 0.05) after 15 months and by 0.8 ± 2.9 (p < 0.001) after 30 months (Fig. 1). The increase in insulin resistance in the overall group was mainly driven by an increase in HOMAIR of 0.49 ± 1.5 (p < 0.01) in the NGT group, and by 1.3 ± 4.3 (p < 0.05) in the T2DM group.
Measurement of liver fat content (CAP) as recorded with sonographic elastography revealed a significantly higher liver fat fraction in overweight subjects with T2DM compared to overweight subjects with NGT or IGT (Fig. 2). When the subjects were divided categorically into those with IR (HOMAIR ≥ 2) or without IR (HOMAIR < 2), those with IR exhibited a significantly higher CAP than insulin-sensitive overweight subjects (297 (286–308) dB/m vs. 270 (241–280) db/m: mean (mean (95% CI); p < 0.001). Linear regression analysis revealed a linear relationship between the HOMAIR score and the CAP measurement (r = 0.32; p < 0.0001). No significant change in CAP could be observed over time.
In accordance with the CAP measurements, FLI, as a clinical score for liver fat content, was significantly higher in overweight subjects with T2DM than in the IGT and NGT groups (Table 1). FLI was significantly higher in those patients with HOMAIR ≥ 2 compared to those with a HOMAIR < 2 (78.6 (75.4–81.8) vs. 67.1 (60.0–74.3); p < 0.0001). The liver fat fraction evaluated by CAP showed a close correlation with FLI (r = 0.50; p < 0.0001).
Due to a change in the study design after the baseline investigations, leptin and adiponectin concentrations were only measured at V2 and V3. Cross-sectional group comparisons for leptin, adiponectin and leptin adiponectin ratio (LAR), without looking into changes over time, were performed. As shown in Table 2, serum leptin concentrations were not significantly different between the three groups of overweight subjects neither at baseline nor at follow-up visits. In accordance with liver fat content and insulin resistance, adiponectin levels were reduced in overweight subjects with T2DM compared to those with NGT or IGT at all visits during the trial. No significant difference could be observed between the subjects with NGT or IGT. When the subjects were categorized according to their CAP measurements in three categories with a liver fat fraction less than 5%, 5 to 33%, or more than 33%, the group of overweight subjects with a liver fat fraction above 33% were found to have significantly higher HOMAIR scores and significantly lower adiponectin levels compared to those groups with a fat fraction of less than 33% or less than 5% (Table 3). An inverse correlation was found between CAP and adiponectin levels (r = -0.15, p < 0.01) and between FLI and adiponectin levels (r = -0.38; p < 0.0001). A linear correlation was observed between CAP and HOMAIR (r = 0.32; p < 0.0001).
While NFS was significantly higher in the overweight subjects with T2DM than in the nondiabetic subjects, no significant difference between the groups could be observed regarding liver stiffness as measured with elastography (E) (Table 1). Neither NFS nor E changed over time in the three overweight or obese groups.
Discussion
According to a recent meta-analysis, it is estimated that approximately one-third of the overall population worldwide is affected by an increase in liver fat content [17]. NAFLD has emerged as the most frequent liver disease, with an estimated prevalence of approximately 25% in the overall population and 70%-80% in high-risk groups, such as obese subjects or patients with T2DM [18,19,20].
Our data obtained from 177 overweight subjects (BMI ≥ 28 kg/m2) are consistent with published data, showing an incidence of NAFLD in approximately 80% of overweight subjects with a BMI ≥ 28 kg/m2 [11]. Insulin resistance and the liver fat fraction were found to be significantly higher in overweight or obese subjects with T2DM than in those with NGT or IGT. No significant difference in insulin resistance or liver fat fraction was observed between obese subjects with NGT or IGT. While insulin resistance continuously increased over 30 months of follow up, liver fat fraction as estimated by CAP or FLI remained almost unchanged. It remains to be established whether this is an indicator of a varying progression rates of insulin resistance and liver fat content over time. NFS and sonographic liver elastography indicated increased liver stiffness and fibrosis in obese subjects with T2DM compared to those without T2DM. No significant change in these parameters could be observed over the period of 30 months.
Insulin resistance is considered to represent a cornerstone pathway in the development of NAFLD and associated hepatic and extrahepatic complications [20, 21]. Our results confirm previous data from Motamed et al. that even in subjects without T2DM, insulin resistance was found to be a strong predictor of NAFLD [22]. In our study, liver fat content was shown to be associated with IR, as indicated by HOMAIR categories. Moreover, regression analysis revealed a strong association between HOMAIR, CAP and the FLI.
Leptin and adiponectin are important adipocytokines that are almost exclusively released from visceral adipocytes. Decreased adiponectin levels and an increased LAR are considered indicators of adipocyte dysfunction and a shift to proinflammatory and profibrotic remodelling of the adipose tissue [23, 24].
In addition to the well-documented low adiponectin levels in overweight subjects [25, 26], our data show even lower adiponectin levels in overweight subjects with T2DM compared to overweight subjects without T2DM. Adiponectin mediates insulin-sensitizing effects by binding to its receptors AdipoR1 and AdipoR2, leading to activation of adenosine monophosphate-dependent kinase (AMPK), PPAR-α, and presumably other yet-unknown signalling pathways [27]. Consistent with these adiponectin signalling pathways, our data reveal an inverse association among adiponectin levels, liver fat content (CAP; FLI) and insulin resistance as expressed by HOMAIR. No association was observed between adiponectin levels and the indicators of liver fibrosis (E; NFS).
No difference in leptin levels were found between the overweight subjects with or without T2DM. This finding is consistent with a publication by Thorand et al., showing a stronger association between adiponectin levels and T2DM compared to leptin levels and T2DM in obese subjects [28].
LAR has been proposed as an even stronger indicator of insulin resistance and T2DM compared to the level of each single adipocytokine alone [24, 29, 30]. In our study, LAR did not provide any additional information on insulin resistance, or the liver fat content compared to single adiponectin levels alone.
The strength of the study is to document the time course of liver fat and liver stiffness in accordance to insulin resistance and glucose control in obese subjects with and without T2DM. Liver fat fraction was found to be elevated in those subjects with higher insulin resistance and with impaired glucose control. Despite a continuous increase in insulin resistance found over a mean of 30 months, no significant change in liver fat content or stiffness could be observed during that time.
There are some important limitations that need to be considered. It might be conceivable that, despite a deterioration of insulin sensitivity, the observational period of 30 months was too short to identify a significant deterioration in liver fat fraction or stiffness. Future studies with longer observational periods should address whether liver fat and stiffness might increase in accordance with an increase in insulin resistance on longer time scales.
The IGT group with 26 subjects was relatively small compared to the NGT group with 88 subjects and the T2DM group with 63 subjects. Therefore, the results obtained for the IGT subgroup should be interpreted with caution.
Leptin and adiponectin levels were only measured at V2 and V3. Therefore, only cross-sectional analysis and no evaluation of changes over time were performed.
Conclusions
In overweight or obese subjects with a BMI ≥ 28 kg/m2, T2DM is associated with increased insulin resistance, increased liver fat and stiffness, and reduced adiponectin levels compared to non-diabetic subjects independent from NGT or IGT. Although insulin sensitivity steadily declined over time in all observed overweight subjects, no significant change in liver fat or liver stiffness was observed over a period of 30 months. Independent of glucose control, insulin resistance, adiponectin levels, and LAR are strong markers for liver steatosis in overweight or obese subjects.
Abbreviations
- CAP:
-
Controlled attenuation parameter
- E:
-
Young’s modulus
- FLI:
-
Fatty liver index
- IGT:
-
Impaired glucose tolerance
- IR:
-
Insulin resistance
- LAR:
-
Leptin/adiponectin ratio
- NAFLD:
-
Nonalcoholic fatty liver disease
- NFS:
-
NAFLD fibrosis score
- NGT:
-
Normal glucose tolerance
- T2DM:
-
Type 2 diabetes mellitus
References
Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019;1(4):312–28. https://doi.org/10.1016/j.jhepr.2019.07.002.
Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71(5):1012–21. https://doi.org/10.1016/j.jhep.2019.06.031.
Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–5. https://doi.org/10.1073/pnas.0904944106.
Cioffi CE, Narayan KMV, Liu K, Uppal K, Jones DP, Tran V, Yu T, Alvarez JA, Bellissimo MP, Maner-Smith KM, Pierpoint B, Caprio S, Santoro N, Vos MB. Hepatic fat is a stronger correlate of key clinical and molecular abnormalities than visceral and abdominal subcutaneous fat in youth. BMJ Open Diabetes Res Care. 2020;8(1). https://doi.org/10.1136/bmjdrc-2019-001126.
Wannamethee SG, Whincup PH, Shaper AG, Lennon L, Sattar N. Γ-glutamyltransferase, hepatic enzymes, and risk of incident heart failure in older men. Arterioscler Thromb Vasc Biol. 2012;32(3):830–5. https://doi.org/10.1161/atvbaha.111.240457.
Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH, Santos RD, Budoff MJ, Nasir K. A systematic review: Burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; Should we care? Atherosclerosis. 2013;230(2):258–67. https://doi.org/10.1016/j.atherosclerosis.2013.07.052.
Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110(7):921–37. https://doi.org/10.1007/s00392-020-01709-7.
Mantovani A, Byrne CD, Benfari G, Bonapace S, Simon TG, Targher G. Risk of heart failure in patients with nonalcoholic fatty liver disease: JACC review topic of the week. J Am Coll Cardiol. 2022;79(2):180–91. https://doi.org/10.1016/j.jacc.2021.11.007.
Mantovani A, Petracca G, Csermely A, Beatrice G, Bonapace S, Rossi A, Tilg H, Byrne CD, Targher G. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut. 2022:gutjnl-2022–327672. https://doi.org/10.1136/gutjnl-2022-327672.
Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021;22(21):11629. https://www.mdpi.com/1422-0067/22/21/11629. Accessed 28 Dec 2023
Forst T, Botz I, Berse M, Voswinkel S, Strempler ME, Baumann S, Schulktz A. Evaluation of non-alcoholic fatty liver disease (NAFLD) in obese adult volunteers according to increasing insulin resistance and loss of glucose control. Curr Res Diabetes Obes J. 2022;15(3); CRDOJ.MS.ID.55914(2022).
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. http://www.ncbi.nlm.nih.gov/pubmed/3899825. Accessed 28 Dec 2023
EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. https://doi.org/10.1186/1471-230x-6-33.
Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010;25(2):49–67. https://doi.org/10.2147/hmer.s7394.
Zhang X, Wong GL, Wong VW. Application of transient elastography in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2020;26(2):128–41. https://doi.org/10.3350/cmh.2019.0001n.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen A-A. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022. https://doi.org/10.1016/S2468-1253(22)00165-0.
Boza C, Riquelme A, Ibanez L, Duarte I, Norero E, Viviani P, Soza A, Fernandez JI, Raddatz A, Guzman S, Arrese M. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15(8):1148–53. https://doi.org/10.1381/0960892055002347.
Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–85. https://doi.org/10.1111/j.1365-2036.2011.04724.x.
Zarghamravanbakhsh P, Frenkel M, Poretsky L. Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metab Open. 2021;12:100149–49. https://doi.org/10.1016/j.metop.2021.100149.
Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ—linking NAFLD and insulin resistance. Endocr Rev. 2019;40(5):1367–93. https://doi.org/10.1210/er.2019-00034.
Motamed B, KohansalVajargah M, Kalantari S, Shafaghi A. HOMA-IR index in non-diabetic patient, a reliable method for early diagnosis of liver steatosis. Caspian J Intern Med. 2022;13(3):519–26. https://doi.org/10.22088/cjim.13.3.519.
Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, Kuzuya H, Shimatsu A, Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27(10):2488–90. https://doi.org/10.2337/diacare.27.10.2488.
Finucane FM, Luan J, Wareham NJ, Sharp SJ, O’Rahilly S, Balkau B, Flyvbjerg A, Walker M, Højlund K, Nolan JJ, Savage DB. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52(11):2345–9. https://doi.org/10.1007/s00125-009-1508-3.
Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10(3):269–79. https://doi.org/10.1111/j.1467-789X.2009.00571.x.
Zhao S, Kusminski CM, Scherer PE. Adiponectin, leptin and cardiovascular disorders. Circ Res. 2021;128(1):136–49. https://doi.org/10.1161/CIRCRESAHA.120.314458.
Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clinica Chimica Acta. 2013;417:80–4. https://doi.org/10.1016/j.cca.2012.12.007.
Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, Koenig W, Thorand B. Leptin, adiponectin, their ratio and risk of coronary heart disease: results from the MONICA/KORA Augsburg study 1984–2002. Atherosclerosis. 2010;209(1):220–5. https://doi.org/10.1016/j.atherosclerosis.2009.08.020.
Oda N, Imamura S, Fujita T, Uchida Y, Inagaki K, Kakizawa H, Hayakawa N, Suzuki A, Takeda J, Horikawa Y, Itoh M. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metabolism. 2008;57(2):268–73. https://doi.org/10.1016/j.metabol.2007.09.011.
Frühbeck G, Catalán V, Rodríguez A, Gómez-Ambrosi J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte. 2018;7(1):57–62. https://doi.org/10.1080/21623945.2017.1402151.
Acknowledgements
The authors thank Marcos Marin-Galiano, M.A.R.C.O. GmbH & Co. KG, Institute for Clinical Research and Statistics, Düsseldorf for data management and statistics and Nadja Faisst, CRS Mannheim for medical writing assistance.
Author information
Authors and Affiliations
Contributions
Thomas Forst was involved in creating the study design, performing the study, and preparing the manuscript.
Sybille Baumann, Matthias Berse, Isabel Botz, and Mares-Elaine Strempler were involved in conducting the study and preparing the manuscript.
Maria Marinez was involved in the discussion of study results and the preparation of the manuscript.
Stephan Voswinkel was responsible for the analysis of the laboratory samples and participated in the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest regarding the contents of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Forst, T., Botz, I., Berse, M. et al. Non-alcoholic fatty liver disease in obese subjects as related to increasing insulin resistance and deteriorating glucose control: Three years of follow-up from a longitudinal survey. J Diabetes Metab Disord 23, 999–1006 (2024). https://doi.org/10.1007/s40200-023-01378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01378-z