Abstract
Purpose
Diabetic foot ulcer (DFU) is one of the most devastating and troublesome consequences of diabetes. The current therapies are not always effective because of the complicated aetiology and interactions of local and systemic components in DFU. However, adjunctive therapy (electromechanical therapy) has become the latest modality in recent years, although there is a lack of significant research to support its utilization as a treatment standard. The purpose of this systematic research was to review the literature on the application of electromechanical therapies in the healing of DFUs.
Methods
For this systematic review, we searched PubMed, Medline, EmBase, the Cochrane library, and Google Scholar for the most current research (1990–2022) on electromechanical therapies for DFUs. We used the PICO method (where P is population, I is intervention, C is comparator/control, and O is outcome for our study) to establish research question with the terms [Electromechanical therapy OR Laser therapy OR photo therapy OR Ultrasound therapy OR Shockwave therapy] AND [diabetic foot ulcers OR diabetes] were used as search criteria. Searches were restricted to English language articles only. Whereas, Cochrane handbook of “Systematic Reviews of Interventions” with critical appraisal for medical and health sciences checklist for systematic review was used for risk of bias assessment. There were 39 publications in this study that were deemed to be acceptable. All the suitably selected studies include 1779 patients.
Results
The meta-analysis of 15 included research articles showed the overall effect was significant (P = 0.0002) thus supporting experimental groups have improvement in the DFUs healing in comparison to the control group.
Conclusion
This systematic review and meta-analysis revealed electromechanical treatments are significantly viable options for patients with DFUs. Electromechanical therapy can considerably reduce treatment ineffectiveness, accelerate healing, and minimize the time it takes for complete ulcer healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is an emerging epidemic with rising incidence, morbidity, and death [1]. Diabetic Foot Ulcer (DFU) is one of the most devastating and troublesome consequences of diabetes and the most significant predictor for lower-extremity amputations [2]. DFU is frequently linked to infection, peripheral neuropathy, and peripheral vascular disease. Nearly 80% of nontraumatic amputations are caused by DFUs, which account for around 35% of the patients in diabetes clinics [2]. Approximately 6% of people worldwide have DFU, and the disease can have up to a 77% five-year mortality rate [2, 3]. According to the International Diabetes Federation, the number of persons with diabetes has constantly increased; currently, there are 643 million cases worldwide, and by 2045, there will be 783 million cases [4].
It is crucial to research techniques and therapies to lessen the burden of the disease in a productive and economical manner since managing DFU continues to be a significant therapeutic problem on a global scale. Due to poor leukocyte chemotaxis and phagocytosis, diminished macrophage activity in the wound matrix, decreased collagen synthesis and deposition, and reduced growth factor release, wound recovery in diabetes patients is often slower than in healthy persons [5, 6]. Diabetes patients have a poor capacity to heal wounds, which makes managing the illness more challenging. Therefore, the development of a treatment for DFUs should take into account a multidisciplinary approach that includes glycaemic management, daily local care, antimicrobials, antiseptics, surgical revascularization, and engineered biological tissues [6, 7].
Surgery debridement, dressings, pressure offloading, vascular assessment, infection treatment, glycaemic control, and patient education make up the standard of care for DFUs [8, 9]. These treatments are not always successful because of the complex aetiology and interaction of local and systemic factors. As a result, it requires a variety of time and cost periods to support the healing process [10]. An optimal adjuvant therapy has yet to be established, which is urgently needed for DFU healing [11]. According to a number of earlier research, nonpharmacological treatments such electrical stimulation [12], low-level laser therapy (LLLT), hyperbaric oxygen therapy [13], and foot off-loading may also be helpful in the healing of DFUs. Additionally, it has been proposed that the use of ultrasound, light therapy, and electrical stimulation will hasten the healing of DFUs by promoting the migration of different cell types and improving wound perfusion [14, 15]. However, an ideal adjuvant therapy has not yet been identified, which is urgently required for the wound healing of DFUs [11]. Due to the dearth of high-quality data that provides solid evidence to support their clinical use, there is a clear need for evidence to substantiate the use of electromechanical therapies (laser therapy, phototherapy, ultrasound therapy, and shockwave therapy) in the management of DFUs [16].
In the current study, we set out to comprehensively review the literature on the application of electromechanical therapies in the healing of DFUs, synthesise the results using meta-analysis of randomised controlled trials (RCTs), and provide clinical guidelines and evidence-based recommendations for the treatment of DFUs in the future.
Material and methods
For this systematic review in November 2022, we searched PubMed, Medline, EmBase, the Cochrane library, and Google Scholar for the most current research (1990–2022) on electromechanical treatments for DFUs. PICO format was followed to design the research question as it is the recommended method by Cochrane and PRISMA guidelines. The terms [Electromechanical therapy OR Laser therapy OR photo therapy OR Ultrasound therapy OR Shockwave therapy] AND [diabetic foot ulcers OR diabetes] were used as search criteria. Searches were restricted to English language articles only. The relevancy of the titles, abstracts, and keywords was checked by two independent reviewers. Publications having titles or abstracts that complied with the requirements for this systematic review were chosen for a more thorough examination. To discover whether there were any other studies that were pertinent, we also went through the reference tracking of bibliographies and manual searches during the first search. The titles and abstracts were evaluated for inclusion by the writers independently. Only studies that satisfied the inclusion criteria were deemed eligible after being located utilising the PRISMA technique and critical appraisal tools (https://jbi.global/critical-appraisal-tools) (Table 1). The risk of bias of included studies was assessed by using an assessment tool of the “Cochrane Handbook for Systematic Reviews of Interventions version” with critical appraisal for medical and health sciences checklist for systematic review.
The data of patients, their age, sex, type of therapy/intervention, duration was extracted. Depending upon the availability of data in the studies we used Standardized Mean Difference (SMD). The statistical analysis (meta-analysis) was performed using Review Manager 5.4 and a 95% confidence interval. Based on the heterogeneity between the studies we selected random or fixed-effect model for meta-analysis. To determine the entire cumulative impact, forest plots were curated.
Quality assessment
The assessment tool covers 7 domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Bias was assessed as “low risk,” “high risk,” or “unclear risk.”
Results
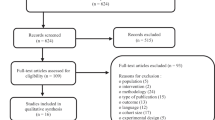
Following the PRISMA guidelines and critical appraisal tools to ensure the quality and consistency of the identified articles, several criteria were used for article eligibility as described in Table 1. After the initial search, 8200 duplicate articles from all researched databases were deleted. Further, 3651 papers were removed from the research after their titles and abstracts were examined. The remaining 449 articles were reviewed and selected by the principal author and co-author based on the set inclusion and exclusion criteria. This study comprised 39 papers that were determined to be eligible (Fig. 1).
The PRISMA flow chart of the literature selection for the meta-analysis. After eliminating any obviously irrelevant information, the authors separately reviewed the research abstracts and full texts to choose which publications to include based on the inclusion and exclusion criteria (Table 1). Any issues or disagreements were discussed by all writers and were resolved
The selected studies include 1779 patients with DFUs. Most of the selected studies have been published in USA (n = 8), followed by Brazil (n = 5) and Egypt (n = 4) as presented in Table 2. While 11 studies reported the use of laser therapy for the treatment of DFUs, 10 studies reported shockwave therapy, 8 studies reported stimulation therapy, 7 reported ultrasound therapy and 3 reported light/phototherapy (Table 2). The mean difference (15.68) for these studies also showed significant difference among experimental and control groups (95% CI, 7.49, 23.87). The overall effect was significant (P = 0.0002) that indicates experimental groups have improvement in the DFUs healing compared to control group. Fifteen studies in the forest plot compared the electromechanical therapies vs placebo/control groups that showed significant difference (P < 0.00001) in heterogeneity among the groups with 98% I2 value (Fig. 2).
Similarly, data from fourteen studies compared the number of healed wounds among experimental and control groups. The overall effect was non-significant (P = 0.12) with odds raio (1.31; 95% CI, 0.93, 1.84) for these studies showing better healing among experimental groups compared to control group. There was also a moderate degree of heterogeneity among these studies (I2 = 68%, P = 0.00001) (Fig. 3).
Discussion
In the current study, we looked at novel therapies used to treat DFUs. After a thorough review, extracorporeal shockwave treatment (ESWT) has been shown through experimental research to accelerate the production of angiogenesis-related growth and proliferation factors, shorten the inflammatory phase, and reduce the risk of wound infection [17,18,19,20,21,22]. Furthermore, by modifying substance P and calcitonin gene-related peptides, ESWT significantly lessens pain in the vicinity of the wound [23]. In 282 patients with chronic wounds who had previously failed conventional treatments, Wolff, Wibmer [18] used ESWT and reported a full cure rate of 74.03% without recrudescence. ESWT was also proven to be effective and well tolerated for treating complex, non-healing, acute, and chronic soft tissue wounds Schaden, Thiele [19]. Thus, ESWT has emerged as a viable first-line treatment for DFU.
In this review, we combined the studies and created a forest plot to compare electromechanical treatments with the placebo/control group. The mean difference for these studies revealed a significant difference between the experimental and control groups, although, the analysis revealed heterogeneity across the groups with a 98% I2 value. Overall, there was a significant difference between the experimental and control groups in how quickly DFUs healed. Our findings support those of earlier research by Butterworth, Walsh [24], Dymarek, Halski [25] and Omar, Gwada [26], which prove the effectiveness of ESWT on chronic wounds.
In a randomised clinical trial by Kaviani, Djavid [27], it was shown that low-level laser treatment (685 nm) cured 8 of 13 (66.6%) ulcers in the experimental group compared to 3 of 9 (33.3%) in the control group receiving sham radiation. However, this finding was not statistically significant. In a Level I investigation, Minatel, Frade [28] reported that healing rates for a group of 7 patients with 10 ulcers treated with combined 660- and 890-nm light were considerably greater at 15-day interval than for a group of 7 patients with 13 ulcers treated with placebo radiation. In a recent RCT (Level II evidence), Petrofsky, Lawson [29] found that electrical stimulation and local dry heat resulted in a statistically significant improvement in DFU healing rates and enhanced blood flow to the surrounding area. ESWT resulted in a substantial reduction in wound size and ulcer healing time when compared to normal therapyOmar, Alghadir [30].
Low-level laser therapy (LLLT), one of the adjuvant treatments, has been identified as a viable mechanism of treatment to hasten the healing of ulcers [31]. The findings of several studies looking into the impact of LLLT on DFU are encouraging. Studies have demonstrated a considerable decrease in the size of the ulcer using LLLT with wavelengths of 632 nm (5 J/cm2; 20 mW)/904 nm (6 J/cm2; 20 mW) and 658 nm (4 J/cm2; 30 mW) [30, 76]. In addition to decrease in DFU pains, considerably higher reductions in ulcer size and the percentage of healing compared to controls have been recorded [32,33,34,35]. However, its therapeutic advantages rely on a number of factors, making it crucial to identify the best parameterization for the efficient treatment of DFU [36]. Additionally, there is a dearth of reliable data that would support the therapeutic use of LLLT in DFU. The benefits of LLLT on DF have been reported in earlier systematic studies [37, 38], however the current review includes significant updates to its clinical effectiveness and improves parameterization for clinical decision-making.
A prior study [38] suggested the LLLT settings of 660 and 890 nm wavelengths, 50 mW/cm2 power density, 2 J/cm2 fluence, 30 s of exposure period, and a distance of 1 cm from the wound. The LLLT parameters used in our study were based on the RCTs showing wavelength: 400–904 nm, power density: 30–180 mW/cm2, and fluence: 2–10 J/cm2. Most of these variables complied with the suggested LLLT settings.
The impacts of LLLT on numerous cellular processes and molecular pathways, such as promoting expression of regulators for cell proliferation, migration, survival, and granulation, were part of the mechanism of LLLT in hastening the healing process of chronic DFU [39]. Additionally, it was discovered that the LLLT group's ulcers had more granulation tissue than the control group [28, 32]. LLLT can increase the expression of essential fibroblast growth factors and induce collagen production in damaged fibroblasts of diabetic mice [40,41,42,43]. Transforming growth factor beta [44], interleukin-1 and interleukin-8 [45], platelet-derived growth factor (PDGF)increased macrophage phagocytic activity [46,47,48,49,50]. The synthesis of collagen and extracellular matrix may be increased, the above-mentioned key cytokines and growth factors may be attracted, and the migration, proliferation, and differentiation of various cell types may all be encouraged by LLLT. All these factors may collectively play significant roles in the healing of DFUs. During LLLT therapy, the epithelium and conjunctive tissues displayed unique and quickly expanding cellular renovation, aiding in the process of tissue healing [51]. According to research by Zhou and colleagues, LLLT can increase the expression of heat shock proteins 70 and 1 in injured tissues, which can then increase the synthesis of growth factors like transforming growth factor-beta and aid in wound healing [52].
Regarding the aspect of potential mechanism, it remains unclear. However, the outcomes of the histopathologic analysis show that ESWT can have both a direct and indirect impact. ESWT might encourage collagen production [19], fibroblastic growth, and angiogenesis by increasing cellular ATP production, which then activates purinergic receptors and Erk1/2 signalling [19, 22, 53]. EWST is therefore believed to have the ability to accelerate the healing process. ESWT, on the other hand, may act as a stimulant of microenvironment metabolism and a promoter of dermal cell development, both of which are necessary for ulcer healing. Additionally, ESWT might promote the production of growth factors, such as fibroblast growth factor, transforming growth factor, insulin-like growth factor-1, platelet-derived growth factor, and vascular endothelial growth factor, which are crucial for DFU wound healing [19, 54]. This would then encourage neovascularization of the tissue and enhance blood perfusion.
In terms of safety, electromechanical therapies are acceptable as non-invasive adjuvant treatments. Electromechanical treatments can have adverse effects during treatment, including temporary skin reddening, mild discomfort, and tiny hematomas. Rarely are serious adverse effects and consequences such as bleeding, thrombosis, muscle injury, and wound infections. These show how superior, safety and tolerance of electromechanical treatments are, as well as their potential to be a workable adjuvant therapy for patients with DFU. Haze, Gavish [55] in a study reported that no device-related adverse events were observed in patients with DFU, and percent closure was significantly greater in the active group compared to sham-treated controls.
Strengths and limitations
The thorough search for evidence, the criteria-based selection of pertinent material, the rigorous assessment of validity, the objective or quantitative summary, and the evidence-based judgments are only a few of this systematic literature review's qualities. The study has several restrictions. The results may only be applicable to people with diabetes and foot ulcers as this meta-analysis only included patients with DFU. Cost-effectiveness was not investigated based on the data available. The included studies' differences in demographic data, baseline ulcer features, and follow-up or treatment periods might possibly have contributed to the heterogeneity observed in the meta- analysis.
Conclusion
According to the findings of the systematic review and meta-analysis, electromechanical treatments are viable and secure choices for individuals with DFUs. Electromechanical therapy can considerably reduce treatment ineffectiveness, speed up healing, and minimize the time it takes for DFUs to heal.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–9.
Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–16.
Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg. 2013;46(1):124–31.
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regener. 1996;4(4):411–20.
Amery C. Growth factors and the management of the diabetic foot. Diabet Med. 2005;22:12–4.
Tsang MW, Wong WKR, Hung CS, Lai K-M, Tang W, Cheung EY, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26(6):1856–61.
Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–65.
Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA, et al. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36:e3266.
Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117(7S):72S-109S.
Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6(1):37.
Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care. 1997;20(3):405–12.
Kessler L, Bilbault P, ORTega F, Grasso C, Passemard R, Stephan D, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26(8):2378–82.
Braun LR, Fisk WA, Lev-Tov H, Kirsner RS, Isseroff RR. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. 2014;15(3):267–81.
Thakral G, LaFontaine J, Najafi B, Talal TK, Kim P, Lavery LA. Electrical stimulation to accelerate wound healing. Diabetic foot & ankle. 2013;4(1):22081.
Liu J, Zhang P, Tian J, Li L, Li J, Tian JH, et al. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2015;(10):CD008474.
Fioramonti P, Onesti MG, Fino P, Fallico N, Scuderi N. Extracorporeal shock wave therapy for the treatment of venous ulcers in the lower limbs. Ann Ital Chir. 2012;83(1):41–4.
Wolff KS, Wibmer A, Pusch M, Prusa AM, Pretterklieber M, Teufelsbauer H, et al. The influence of comorbidities and etiologies on the success of extracorporeal shock wave therapy for chronic soft tissue wounds: midterm results. Ultrasound Med Biol. 2011;37(7):1111–9.
Schaden W, Thiele R, Kölpl C, Pusch M, Nissan A, Attinger CE, et al. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J Surg Res. 2007;143(1):1–12.
Vetrano M, d’Alessandro F, Torrisi MR, Ferretti A, Vulpiani MC, Visco V. Extracorporeal shock wave therapy promotes cell proliferation and collagen synthesis of primary cultured human tenocytes. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2159–68.
Kuo YR, Wang CT, Wang FS, Chiang YC, Wang CJ. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regener. 2009;17(4):522–30.
Goertz O, Hauser J, Hirsch T, von der Lohe L, Kolbenschlag J, Stricker I, et al. Short-term effects of extracorporeal shock waves on microcirculation. J Surg Res. 2015;194(1):304–11.
Zhang L, Weng C, Zhao Z, Fu X. Extracorporeal shock wave therapy for chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regener. 2017;25(4):697–706.
Butterworth PA, Walsh TP, Pennisi YD, Chesne AD, Schmitz C, Nancarrow SA. The effectiveness of extracorporeal shock wave therapy for the treatment of lower limb ulceration: a systematic review. J Foot Ankle Res. 2015;8(1):1–9.
Dymarek R, Halski T, Ptaszkowski K, Slupska L, Rosinczuk J, Taradaj J. Extracorporeal shock wave therapy as an adjunct wound treatment: a systematic review of the literature. Ostomy Wound Manage. 2014;60(7):26–39.
Omar MT, Gwada RF, Shaheen AA, Saggini R. Extracorporeal shockwave therapy for the treatment of chronic wound of lower extremity: current perspective and systematic review. Int Wound J. 2017;14(6):898–908.
Kaviani A, Djavid GE, Ataie-Fashtami L, Fateh M, Ghodsi M, Salami M, et al. A randomized clinical trial on the effect of low-level laser therapy on chronic diabetic foot wound healing: a preliminary report. Photomed Laser Surg. 2011;29(2):109–14.
Minatel DG, Frade MAC, França SC, Enwemeka CS. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg Med. 2009;41(6):433–41.
Petrofsky JS, Lawson D, Berk L, Suh H. Enhanced healing of diabetic foot ulcers using local heat and electrical stimulation for 30 min three times per week. J Diabetes. 2010;2(1):41–6.
Omar MT, Alghadir A, Al-Wahhabi KK, Al-Askar AB. Efficacy of shock wave therapy on chronic diabetic foot ulcer: a single-blinded randomized controlled clinical trial. Diabetes Res Clin Pract. 2014;106(3):548–54.
Andrade FdSdSD, Clark RMdO, Ferreira ML. Effects of low-level laser therapy on wound healing. Rev Col Bras Cirurgiões. 2014;41:129–33.
Mathur R, Sahu K, Saraf S, Patheja P, Khan F, Gupta P. Low-level laser therapy as an adjunct to conventional therapy in the treatment of diabetic foot ulcers. Lasers Med Sci. 2017;32(2):275–82.
de Alencar Fonseca Santos J, Campelo MBD, de Oliveira RA, Nicolau RA, Rezende VEA, Arisawa EÂL. Effects of low-power light therapy on the tissue repair process of chronic wounds in diabetic feet. Photomed Laser Surg. 2018;36(6):298–304.
Porto Feitosa MC, Machado de Carvalho AF, Feitosa VC, de Oliveira RA, Machado de Freitas Coelho NP, Nogueira Rebelo VC, et al. Pain and Quality of life of diabetic patients with ulcers, before and after treatment with low intensity laser therapy and Hellantus Annus oil. Mundo da saúde. 2017;41(1):18–29.
Carvalho AFMd, Feitosa MCP, Coelho NPMdF, Rebêlo VCN, Castro JGd, Sousa PRGd, et al. Low-level laser therapy and Calendula officinalis in repairing diabetic foot ulcers. Revista da Escola de Enfermagem da USP. 2016;50:0628–34.
Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regen Med. 2016;11(1):107–22.
Li S, Wang C, Wang B, Liu L, Tang L, Liu D, et al. Efficacy of low-level light therapy for treatment of diabetic foot ulcer: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2018;143:215–24.
Tchanque-Fossuo CN, Ho D, Dahle SE, Koo E, Li CS, Isseroff RR, et al. A systematic review of low-level light therapy for treatment of diabetic foot ulcer. Wound Repair Regener. 2016;24(2):418–26.
Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg. 2012;30(11):617–36.
Ayuk SM, Houreld NN, Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol Ther. 2012;14(12):1110–7.
Aparecida Da Silva A, Leal-Junior ECP, Alves ACA, Rambo CS, Dos Santos SA, Vieira RP, et al. Wound-healing effects of low-level laser therapy in diabetic rats involve the modulation of MMP-2 and MMP-9 and the redistribution of collagen types I and III. J Cosmetic Laser Ther. 2013;15(4):210–6.
Byrnes KR, Barna L, Chenault VM, Waynant RW, Ilev IK, Longo L, et al. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed Laser Ther. 2004;22(4):281–90.
Yu H-S, Wu C-S, Kao Y-H, Chiou M-H, Yu C-L. Helium–neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo. J Investig Dermatol. 2003;120(1):56–64.
Safavi SM, Kazemi B, Esmaeili M, Fallah A, Modarresi A, Mir M. Effects of low-level He–Ne laser irradiation on the gene expression of IL-1β, TNF-α, IFN-γ, TGF-β, bFGF, and PDGF in rat’s gingiva. Lasers Med Sci. 2008;23(3):331–5.
Yu H-S, Chang K-L, Yu C-L, Chen J-W, Chen G-S. Low-energy helium-neon laser irradiation stimulates interleukin-1α and interleukin-8 release from cultured human keratinocytes. J Investig Dermatol. 1996;107(4):593–6.
Mester E, Ludany G, Sellyei M, Szende B, Gyenes G, Tota G. Studies on the inhibiting and activating effects of laser beams. Langenbecks Arch Chir. 1968;322:1022–7.
Dube A, Bansal H, Gupta P. Modulation of macrophage structure and function by low level He-Ne laser irradiation. Photochem Photobiol Sci. 2003;2(8):851–5.
Hemvani N, Chitnis DS, Bhagwanani NS. Helium-neon and nitrogen laser irradiation accelerates the phagocytic activity of human monocytes. Photomed Laser Surg. 2005;23(6):571–4.
Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers Surg Med. 1989;9(5):497–505.
Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122(4):532–5.
Feitosa MCP, Carvalho AFMd, Feitosa VC, Coelho IM, Oliveira RAd, Arisawa EÂL. Effects of the Low-Level Laser Therapy (LLLT) in the process of healing diabetic foot ulcers. Acta Cirurgica Bras. 2015;30:852–7.
Zhou J-d, Luo C-q, Xie H-q, Nie X-m, Zhao Y-z, Wang S-h, et al. Increased expression of heat shock protein 70 and heat shock factor 1 in chronic dermal ulcer tissues treated with laser-aided therapy. Chin Med J. 2008;121(14):1269–73.
Weihs AM, Fuchs C, Teuschl AH, Hartinger J, Slezak P, Mittermayr R, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014;289(39):27090–104.
Hayashi D, Kawakami K, Ito K, Ishii K, Tanno H, Imai Y, et al. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: A critical role of endothelial nitric oxide synthase. Wound Repair Regener. 2012;20(6):887–95.
Haze A, Gavish L, Elishoov O, Shorka D, Tsohar T, Gellman YN, et al. Treatment of diabetic foot ulcers in a frail population with severe co-morbidities using at-home photobiomodulation laser therapy: a double-blind, randomized, sham-controlled pilot clinical study. Lasers Med Sci. 2022;37(2):919–28.
Gao H, Chen J, Zhao Z, Wang G. A combination of ultrasonic debridement and topical cortex phellodendri compound fluid in patients with diabetic foot ulcers. Medicine (Baltimore). 2022;101(32):e29604.
Zulbaran-Rojas A, Park C, El-Refaei N, Lepow B, Najafi B. Home-Based Electrical Stimulation to Accelerate Wound Healing—A Double-Blinded Randomized Control Trial. J Diabetes Sci Technol. 2023;17(1):15–24.
Lázaro-Martínez JL, Álvaro-Afonso FJ, Sevillano-Fernández D, García-Álvarez Y, Sanz-Corbalan I, García-Morales E. Cellular proliferation, dermal repair, and microbiological effectiveness of ultrasound-assisted wound debridement (UAW) versus standard wound treatment in complicated diabetic foot ulcers (DFU): an open-label randomized controlled trial. J Clin Med. 2020;9(12):4032.
Vitoriano NAM, Mont’Alverne DGB, Martins MIS, Silva PS, Martins CA, Teixeira HD, et al. Comparative study on laser and LED influence on tissue repair and improvement of neuropathic symptoms during the treatment of diabetic ulcers. Lasers Med Sci. 2019;34(7):1365–71.
Rastogi A, Bhansali A, Ramachandran S. Efficacy and safety of low-frequency, noncontact airborne ultrasound therapy (Glybetac) for neuropathic diabetic foot ulcers: a randomized, double-blind, sham-control study. Int J Low Extrem Wounds. 2019;18(1):81–8.
Abd El Fattah AM, Shaaban M, Gawish H, El Mashad N, Dawood AED. Effect of ultrasound-assisted debridement on wound healing and infection outcomes in diabetic foot. Menoufia Med J. 2018;31(2):462.
Bajpai A, Nadkarni S, Neidrauer M, Weingarten MS, Lewin PA, Spiller KL. Effects of non-thermal, non-cavitational ultrasound exposure on human diabetic ulcer healing and inflammatory gene expression in a pilot study. Ultrasound Med Biol. 2018;44(9):2043–9.
Michailidis L, Bergin SM, Haines TP, Williams CM. Healing rates in diabetes-related foot ulcers using low frequency ultrasonic debridement versus non-surgical sharps debridement: a randomised controlled trial. BMC Res Notes. 2018;11(1):1–5.
Snyder R, Galiano R, Mayer P, Rogers LC, Alvarez O, Investigators ST. Diabetic foot ulcer treatment with focused shockwave therapy: two multicentre, prospective, controlled, double-blinded, randomised phase III clinical trials. J Wound Care. 2018;27(12):822–36.
Tantawy SA, Abdelbasset WK, Kamel DM, Alrawaili SM. A randomized controlled trial comparing helium-neon laser therapy and infrared laser therapy in patients with diabetic foot ulcer. Lasers Med Sci. 2018;33(9):1901–6.
Asadi MR, Torkaman G, Hedayati M, Mohajeri-Tehrani MR, Ahmadi M, Gohardani RF. Angiogenic effects of low-intensity cathodal direct current on ischemic diabetic foot ulcers: a randomized controlled trial. Diabetes Res Clin Pract. 2017;127:147–55.
El Rasheed NAA, Mahmoud NF, Hamada HA, El Khatib A. Pulsed electromagnetic fields versus laser therapy on enhancing recovery of diabetic foot ulcer: A single blind randomized controlled trial. Biomedical Research 2017;28(19): 8509–8514.
Srilestari A, Nareswari I, Simadibrata C, Tarigan TJ. Effectiveness of combined laser-puncture and conventional wound care to accelerate diabetic foot ulcer healing. Med J Indonesia. 2017;26(1):26–34.
Jeppesen S, Yderstraede K, Rasmussen B, Hanna M, Lund L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: a prospective randomised trial. J Wound Care. 2016;25(11):641–9.
Sandoval Ortíz MC, Herrera Villabona E, Camargo Lemos DM, Castellanos R. Effects of low level laser therapy and high voltage stimulation on diabetic wound healing. Rev Univ Ind Santander Salud. 2014;46(2):107–17.
Liani M, Trabassi E, Cusaro C, Zoppis E, Maduli E, Pezzato R, et al. Effects of a pulsatile electrostatic field on ischemic injury to the diabetic foot: evaluation of refractory ulcers. Primary Care Diabetes. 2014;8(3):244–9.
Mohajeri-Tehrani MR, Annabestani Z. Effect of low-intensity direct current on expression of vascular endothelial growth factor and nitric oxide in diabetic foot ulcers. J Rehabil Res Dev. 2014;51(5):815.
Nossair AA, Eid MM, Salama AB. Advanced protocol of shock wave therapy for diabetic foot ulcer. J Am Sci. 2013;9:633–8.
Ottomann C, Stojadinovic A, Lavin PT, Gannon FH, Heggeness MH, Thiele R, et al. Prospective randomized phase II Trial of accelerated reepithelialization of superficial second-degree burn wounds using extracorporeal shock wave therapy. Ann Surg. 2012;255(1):23–9.
Kajagar BM, Godhi AS, Pandit A, Khatri S. Efficacy of low level laser therapy on wound healing in patients with chronic diabetic foot ulcers—a randomised control trial. Indian J Surg. 2012;74(5):359–63.
Landau Z, Migdal M, Lipovsky A, Lubart R. Visible light-induced healing of diabetic or venous foot ulcers: a placebo-controlled double-blind study. Photomed Laser Surg. 2011;29(6):399–404.
Wang C-J, Wu R-W, Yang Y-J. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Res Clin Pract. 2011;92(2):187–93.
Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafuri S, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskelet Disord. 2009;10(1):1–8.
Wang C-J, Kuo Y-R, Wu R-W, Liu R-T, Hsu C-S, Wang F-S, et al. Extracorporeal shockwave treatment for chronic diabetic foot ulcers. J Surg Res. 2009;152(1):96–103.
Saggini R, Figus A, Troccola A, Cocco V, Saggini A, Scuderi N. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med Biol. 2008;34(8):1261–71.
Kavros SJ, Miller JL, Hanna SW. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo clinic experience, 2004–2006. Adv Skin Wound Care. 2007;20(4):221–6.
Petrofsky JS, Lawson D, Suh HJ, Rossi C, Zapata K, Broadwell E, et al. The influence of local versus global heat on the healing of chronic wounds in patients with diabetes. Diabetes Technol Ther. 2007;9(6):535–44.
Peters EJ, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: a randomized clinical trial. Arch Phys Med Rehabil. 2001;82(6):721–5.
Lundeberg TC, Eriksson SV, Malm M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann Plast Surg. 1992;29(4):328–31.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rathnayake, A., Saboo, A., Vangaveti, V. et al. Electromechanical therapy in diabetic foot ulcers patients: A systematic review and meta-analysis. J Diabetes Metab Disord 22, 967–984 (2023). https://doi.org/10.1007/s40200-023-01240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-023-01240-2