Abstract
Background
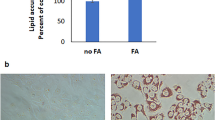
In hepatic damage, Hepatic stellate cells (HSCs) become active, proliferate, and change to myofibroblasts. Increasing the fibrogenic genes, such as Transforming growth factor-β (TGF-β), Alpha Smooth Muscle Actin (α-SMA), and Collagen1 α (COL 1α) show that the activation of HSCs can lead to hepatic fibrosis.
Purpose
These days people consume much cholesterol, palmitic acid, and glucose which can have adverse effects on an individuals’ health, but their influences on activating human HSCs and inducing liver fibrosis have not been assessed. Our purpose is to investigate the effects of these three main and abundant ingredients in the diet on the activation of human HSCs and inducing liver fibrosis.
Methods
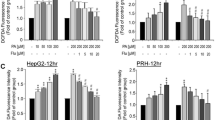
To measure cholesterol, palmitic acid, and glucose cytotoxic effects on the viability of the cells, the MTT technique was used. Then the treated cells were incubated in media containing cholesterol, palmitic acid, and glucose with different concentrations for 24 h. At last, the α-SMA, COL 1α, and TGF-β, genes mRNA expression were measured by real-time PCR.
Results and Conclusions
Our results demonstrated that high concentrations of cholesterol and palmitic acid can activate human HSCs that lead to an increase in the mRNA expressions of fibrogenic genes. Thus, controlling fat intaking and knowing its mechanism is crucial to prevent and attenuate hepatic fibrosis.



Similar content being viewed by others
Data availability
The data in this study are present from the corresponding author if requested.
Abbreviations
- TGF-β:
-
1-Transforming growth factor-β
- α-SMA:
-
2-Alpha Smooth Muscle Actin
- COL 1α:
-
3-Collagen1 α
- HSCS:
-
Human hepatic stellate cells
- ECM:
-
Extracellular matrix
- NAFLD:
-
Non-alcoholic fatty liver disease
- MTT assay:
-
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2 H-tetrazolium bromide
- FFA:
-
free fatty acids
- TGs:
-
triglycerides
References
Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875.
Friedman SL, Maher JJ, Bissell DM. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. 2000, Wiley Online Library.
Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39(4):S158-61.
Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Ann Hepatol. 2017;16(1):48–56.
Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and management. Am Family Phys. 2019;100(12):759–70.
Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18(1):179–90.
Winau F, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26(1):117–29.
Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiology-Gastrointestinal Liver Physiol. 2000;279(1):G7-11.
Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67.
Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-β signaling pathway in hepatic fibrosis. Liver Int. 2006;26(1):8–22.
Bissell D, et al. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Investig. 1995;96(1):447–55.
Bissell DM, Roulot D, George J. Transforming growth factor β and the liver. Hepatology. 2001;34(5):859–67.
Martin M, Lefaix J-L, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiation Oncology* Biology* Phys. 2000;47(2):277–90.
Kweon Y-O, et al. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J Hepatol. 2003;39(1):38–46.
Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of a high-cholesterol diet resulted in hepatic steatosis, focal nodular hyperplasia and fibrosis in non-obese mice. Br J Nutr. 2010;103(3):378–85.
Kainuma M, et al. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. J Gastroenterol. 2006;41(10):971–80.
Teratani T, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142(1):152–64.
Nehra V, et al. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46(11):2347–52.
Seki S, et al. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37(1):56–62.
Pérez-Carreras M, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007.
Reeves HL, et al. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25(5):677–83.
Vazquez-Jimenez JG, et al. Palmitic acid but not palmitoleic acid induces insulin resistance in a human endothelial cell line by decreasing SERCA pump expression. Cell Signal. 2016;28(1):53–9.
Kleinfeld AM, et al. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78(12):1350–4.
Takkunen MJ, et al. Longitudinal associations of serum fatty acid composition with type 2 diabetes risk and markers of insulin secretion and sensitivity in the Finnish Diabetes Prevention Study. Eur J Nutr. 2016;55(3):967–79.
Dong Z, et al. Palmitic acid stimulates NLRP3 inflammasome activation through TLR4-NF-κB signal pathway in hepatic stellate cells. Ann Trans Med. 2020;8(5).
Kiss K, et al. Chronic hyperglycaemia induced alterations of hepatic stellate cells differ from the effect of TGFB1, and point toward metabolic stress. Pathol Oncol Res. 2020;26(1):291–9.
Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3):677.
Zhou L, et al. miR-185 inhibits fibrogenic activation of hepatic stellate cells and prevents liver fibrosis. Mol Therapy-Nucleic Acids. 2018;10:91–102.
Iredale J. Defining therapeutic targets for liver fibrosis: exploiting the biology of inflammation and repair. Pharmacol Res. 2008;58(2):129–36.
Liu Z, et al. Transforming growth factor β (TGFβ) cross-talk with the unfolded protein response is critical for hepatic stellate cell activation. J Biol Chem. 2019;294(9):3137–51.
Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepato-Biliary‐Pancreatic Sci. 2015;22(7):512–8.
Carpino G, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37(5):349–56.
Tomita K, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59(1):154–69.
Meissen JK, et al. Temporal metabolomic responses of cultured HepG2 liver cells to high fructose and high glucose exposures. Metabolomics. 2015;11(3):707–21.
Wobser H, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19(8):996–1005.
Fabregat I, et al. TGF-β signalling and liver disease. FEBS J. 2016;283(12):2219–32.
Acknowledgements
The authors thank the University of Medical Sciences of the Ahvaz Jundishapur for the financial support of the study.
Funding
Medical Sciences Ahvaz Jundishapur University has supported this study (grant number. HLRC- CMRC-0009). The funding has not affected any steps of the research.
Author information
Authors and Affiliations
Contributions
GHM planned the research. ESH did assay. RA analyses the obtained results. SSB and ESH wrote the manuscript and revised it. FA and SAZ interpreted the data. All authors confirmed the final article.
Corresponding author
Ethics declarations
Ethics approval to participate
Ethical clearance was not needed and not sought from the Review Board of Ahwaz Jundishapur University of Medical Sciences, because the study was done on cell lines in vitro, and did not use human samples.
Publication consent
No applicable.
Competing interests
The authors report no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadzadeh, G., Afarin, R., Bavarsad, S.S. et al. Comparison of the effects of cholesterol, palmitic acid, and glucose on activation of human hepatic stellate cells to induce liver fibrosis. J Diabetes Metab Disord 21, 1531–1538 (2022). https://doi.org/10.1007/s40200-022-01095-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01095-z