Abstract
Background
Diabetes mellitus is a metabolic disorder characterised by chronic hyperglycemia. The present research work aimed to evaluate the hypoglycaemic, hypolipidemic and antioxidant effects of leafy stems of Cissus polyantha Gilg & Brandt in insulin resistant rats.
Methods
The oral glucose tolerance test (OGTT) was performed in normal rats. Hyperglycemia was induced for 8 days by a daily subcutaneous injection of dexamethasone (1 mg/kg) one hour after pretreatment of animals with metformin (40 mg/kg) and C. polyantha extract (111, 222 and 444 mg/kg). Body weight, blood glucose, insulin level, lipid profile, insulin biomarkers, cardiovascular indices and oxidative stress biomarkers were evaluated.
Results
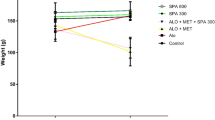
For OGTT, the extract (444 mg/kg) produced a significant drop in blood sugar at the 60th (p < 0.01), 90th (p < 0.01) and 120th min (p < 0.05). Morever, the extract at doses of 222 and 111 mg/kg significantly reduced blood sugar at the 60th (p < 0.01) and 90th min (p < 0.05) respectively. Otherwise, C. polyantha (444 and 222 mg/kg) significantly (p < 0.001) increased body weight and decreased blood sugar on the 4th and 8th days of treatment in insulin resistant rats. The extract also significantly decreased (p < 0.001) serum insulin level, hyperlipidemia, insulin resistance index and cardiovascular indices, and increased gluthathione level, and superoxide dismutase and catalase activity.
Conclusion
The aqueous extract of Cissus polyantha leafy stems (AECPLS) possess hypoglycemic, hypolipidemic and antioxidant activities that could justify its use in traditional medicine for the prevention and treatment of diabetes mellitus and its complications.

Similar content being viewed by others
References
Reaven GM. The insulin resistance syndrome. Curr Atheroscler Rep. 2003;5:364–71.
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54.
Nelly Rivera-Yañez, Rodriguez-Canales M, Nieto-Yañez O, Jimenez-Estrada M, Ibarra-Barajas M, Canales-Martinez M, Rodriguez-Monroy MA. Hypoglycaemic and antioxidant effects of propolis of Chihuahua in a model of experimental diabetes. Evid Based Complement Altern Med. 2018;4360356:10. https://doi.org/10.1155/2018/4360356.
International Diabete Federation. Global overview. Diabetes Atlas. 8th ed. 2017. p. 150.
Cheng AYY, Funtus IG. Oral antihyperglycaemic therapy for type 2 diabetes mellitus. Can Med Assoc J. 2005;172:213–26.
Matheka DM, Demaio AR. Complementary and alternative medicine use among diabetic patients in Africa: a Kenyan perspective. Pan Afr Med J. 2013;15:110.
Akhtar FM, Ali MR. Study of the antidiabetic effect of a compound medicinal plant prescription in normal and diabetic rabbit. J Pakistan Med Assoc. 1984;34:239–44.
Lanjhiyana S, Garabadu D, Ahirwar D, et al. Hypoglycemic activity studies on root extracts of Murraya koenigii root in Alloxan-induced diabetic rats. J Nat Prod Plant Resour. 2011;1:91–104.
Patil AA, Koli AS, Darshana A, Patil B, Narayane CV, Phatak VAA. Evaluation of effect of aqueous slurry of Curculigo orchioides aertn rhizome in streptozotocin induced diabetic rats. J Pharm Res. 2013;7:747–5.
Burkill H. The useful plants of west tropical africa. Royal Botanic Garden Kew. 2000;5:290.
Sani Y, Musa A, Pateh U, et al. Phytochemical screening and preliminary evaluation of analgesic and anti-inflammatory activities of the methanol root extract of Cissus polyantha. BAJOPAS. 2014;7:19–23.
Mahamad AT, Miaffo D, Poualeu Kamani S, Kamanyi A, Wansi SL. Antioxidant properties and digestive enzyme inhibitory activity of the aqueous extract from leafy stems of Cissus polyantha. Evid Based Complement Altern Med. 2019;2019:1–7. https://doi.org/10.1155/2019/7384532.
Savithramma N, Rao ML, Rukmini K, Devi PS. Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int J of Chem Tech Res. 2011;3:1394–402.
Shannon R, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Federation Am Societies Experimental Biol J. 2008;22:659–61.
Fofié KC, Nguelefack-Mbuyo EP, Tsabang N, Kamanyi A, Nguelefack BT. Hypoglycemic properties of the aqueous extract from the stem bark of Ceiba pentandra in dexamethasone-induced insulin resistant rats. Evid Based Complement Altern Med. 2018;2018:1–11. https://doi.org/10.1155/2018/4234981.
Matthews DR, Hosker JP, Rudenski AS, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–8.
Ma Y, Wang Y, Huang Q, Ren Q, Chen S, Zhang A, et al. Impaired β cell function in Chinese newly diagnosed type 2 diabetes mellitus with hyperlipidemia. J Diabetes Res. 2014;493039:1–6.
Li B, Lin W, Lin N, Dong X, Liu L. Study of the correlation between serum ferritin levels and the aggregation of metabolic disorders in non-diabetic elderly patients. Exp Ther Med. 2014;7:1671–6.
Trinder P. Quantitative in vitro determination of cholesterol in serum and plasma. Ann Clin Biochem. 1969;6:24–7.
Weiber DA, Warnick GR. Measurement of high-density lipoprotein cholesterol. Handbook of lipoprotein testing. Washington: AACC Press; 1997. p. 44–127.
Cole TG, Klotzsch SG, Mcnamara J. Measurement of triglyceride concentration. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. Washington: AACC Press; 1997. p. 115–26.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentrationof low-density lipoprotein cholesterol in plasma without use of the preparativeultracentrifuge. Clin Chem. 1972;18:499–502.
Rafieian-Kopaei M, Shahinfard N, Rouhi Boroujeni H, Gharipour M, Darvishzadeh-Boroujeni P. Effects of Ferulago angulata extract on serum lipids and lipid peroxidation. Evid Based Complement Alternat Med. 2014; 680856:4. https://doi.org/10.1155/2014/680856.
Kang HT, Kim JK, Kim JY, Linton JA, Yoon JH, Koh SB. Independent association of TG/ HDL-c with urinary albumin excretion in normotensive subjects in a rural Korean population. Clin Chim Acta. 2012;413:319–24.
Barter P, Gotto AM, La Rosa JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10.
Quantanilha AT, Packer L, Szyszlo DJA, Racnelly TL, Davies KJA. Membrane effects of vitamin E deficiency bioenergetic and surface charge density of skeletal muscle and liver mitochondria. Ann N Y Acad Sci. 1982;393:32–47.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94.
Misra HP, Fridovish I. The role of superoxide anion in the autoxidation of epinephrine and a sample assay for superoxide dismustase. J Biol Chem. 1972;247:3170–5.
Sehirli O, Tozan A, Omurtag GZ, Cetine S, Contuk G, Gedik N, et al. Protective effects of resveratol against naphtylene-induced oxidative stress in mica. Ecoto Environ Safe. 2008;71:301–8.
Stump CS, Clark SE, Sowers JR. Oxidative stress in insulin-resistant conditions: cardiovascular implications. Treat Endocrinol. 2005;4:343–51.
Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607.
Clark CM Jr, Lee DA. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210–7.
Cohen A, Horton ES. Progress in the treatment of type 2 diabetes: new pharmacologic approaches to improve glycemic control. Curr Med Res Opin. 2007;23:905–17.
Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94.
Gray AM, Flatt PR. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br J Nutr. 1997;78:325–34.
Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275–84.
Sani Y, Musa A, Yaro A, Sani M, Amoley A, Magaji MG. Phytochemical screening and evaluation of analgesic and anti-inflammatory activities of the methanol leaf extract of Cissus polyantha. J Med Sci. 2013;10:1–5.
Pari L, Latha M. Antidiabetic effect of Scoparia dulcis: effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005;24:13–26.
Valsa AK, Sudheesh S, Vijayalakshmi NR. Effect of catechin on carbohydrate metabolism. Indian J Biochem Biophys. 1997;34:406–8.
Mahendran P, Shyamala Devi CS. Effect of Garcinia cambogia extract on lipids and lipoprotein composition in dexamethasone administered rats. Indian J Physiol Pharmacol. 2001;45:345–50.
Rossetti L, DeFronzo RA, Gherzi R, et al. Effects of metformin treatment on insulin action in diabetic rats: in vivo and in vitro correlations. Metabolism. 1990;39:425–35.
Ovalle-Magallanes B, Medina-Campos ON, Pedraza-Chaverri J, Mata R. Hypoglycemic and anti hyperglycemic effects of phytopreparations and limonoids from Swieteniahumilis. Phytochemistry. 2015;110:111–9.
Ashpak Tamboli M, Sujit Karpe T, Sohrab Shaikh A, Anil Manikrao M, Dattatraya KV. Hypoglycemic activity of extracts of Oroxylum indicum (L.) vent roots in animal models. Pharmacologyonline. 2011;2:890–9.
Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibitsinsulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism. 1998;47:3–6.
Bailey CJ. Metformin: a useful adjunct to insulin therapy? Diabet Med. 2000;17:83–4.
Jyoti M, Vihas TV, Ravikumar A, Sarita G. Glucose lowering effect of aqueous extract of Enicostemma littorale Blume in diabetes: a possible mechanism of action. J Ethnopharmacol. 2002;81:317–20.
Schacke H, Rehwinkel A. Dissociated glucocorticoid receptor ligands. Curr Opin Investig Drugs. 2004;5:524–8.
Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–22.
Daly ME, Vale C, Walker M, Alberti KG, Mathers JC. Dietary carbohydrates and insulin sensitivity: a review of the evidence and clinical implications. Am J Clin Nutr. 1997;66:1072–85.
Defronzo RA, Ferrannini E. Insulin resistance: a mullifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabete Care. 1991;14:173–94.
Geohas J, Daly A, Juturu V, Finch M, Komorowski JR. Chromium picolinate and biotin combination reduces atherogenic index of plasma in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized clinical trial. Am J Med Sci. 2007;333:145–53.
Cooper ME. Pathogenesis, prevention, and treatment of diabetic neuropathy. Lancet. 1998;352:213–9.
Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev. 1998;19:477–90.
Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin induced diabetic rats. Mol Cell Biochem. 2003;243:147–52.
Mates JM, Perez-gomez C, Nunez De Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603.
Hassan HM. Biosynthesis and regulation of superoxide dismustases. Free Radic Biol Med. 1988;5:377–85.
Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis. 2007;115:81–103.
Irudayaraj SS, Sunil C, Duraipandiyan V, Ignacimuthu S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) lam. Leaves in streptozotocin induced diabetic rats. J Ethnopharmacol. 2012;143:515–23.
Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol Res. 2000;33:55–64.
Rajat B. Screening and quantification of phytochemicals and evaluation of antioxidant activity of Albizia chinensis (Vang): one of the tree foliages commonly utilized for feeding to cattle and buffaloes in Mizoram. Int J Curr Microbiol App Sci. 2015;4:305–13.
Acknowledgments
This work was carried at the Laboratory of Department of Biological Science, Faculty ofSciences, University of Maroua, Cameroon. The authors are grateful to the Head of thislaboratory for providing the facilities, and M. MOUSSA Abame for the purchase of the reagents.
Data of availability
The data analyzed and materials used in this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
MTA and DA proposed the plant material, harvested and prepared the crude extract. MTA, DM and MO conducted the different tests in laboratory. DM and SKP analyzed the data and drafted the article. AK and SLW corrected the final manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were handled according to the Ethic Committee of the Faculty of Sciences of the University of Maroua (Ref. N°14/0261/ Uma/D/FS/VD-RC), Cameroon. The Animal protocol were accomplished in accordance with the guidelines of Cameroonian bioethics committee (reg N°.FWA-IRB00001954) and NIH- Care and Use of Laboratory Animals manual (8th Edition).
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflicts interest related to this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahamad, A.T., Miaffo, D., Poualeu Kamani, S.L. et al. Glucose, lipid and oxidative stress lowering activity of the aqueous extract from leafy stems of Cissus polyantha Gilg & Brandt in dexamethasone-induced hyperglycemia in rats. J Diabetes Metab Disord 19, 1527–1535 (2020). https://doi.org/10.1007/s40200-020-00687-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00687-x