Abstract
Background
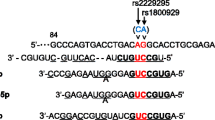
Type 2 diabetes mellitus (T2DM) is a heterogenic disease with increasing incidence. The SLC30A8 gene encodes an islet zinc transporter (ZnT8), and its variants have been associated with glucose and pro-insulin levels. This study was aimed to examine the effects of a missense variant (rs13266634 C/T), and two 3’UTR variants (rs2466294 C/G and rs2466293 T/C) in SLC30A8 gene on T2DM risk in a south-east Iranian population.
Methods
In this experiment, 450 patients diagnosed with T2DM and 453 healthy subjects from the same geographic area were enrolled. Genotypes were amplified using the ARMS–PCR method. In silico analyses were performed to determine the effects of the variants on the local structure of mRNA, splicing patterns, and potential miRNA-gene interactions as well.
Results
Significant differences were noticed between cases and controls regarding the genotypic and allelic distribution of the studied variants. As regards rs2466293 and rs2466294 variants, enhanced risk of T2DM was found under allelic, dominant, recessive, and codominant models (OR > 1). Besides, different genetic models of rs13266634 were associated with decreased risk of T2DM (OR < 1). Bioinformatics analyses indicated that the rs2466293 variant might influence the binding of some miRNAs, while the G-allele of rs2466294 decreased the stability of SLC30A8-mRNA.
Conclusions
In our population, both SNPs in the 3′-untranslated region of SLC30A8 increased the risk of T2DM, while the rs13266634 variant showed a protective association against T2DM susceptibility. Investigating the effects of other variants in this gene or other ZnTs can further indicate such associations in subjects from the same ethnicity.




Similar content being viewed by others
References
Lu G, Teng X, Zheng Z, Zhang R, Peng L, Zheng F, et al. Overexpression of a glucokinase point mutant in the treatment of diabetes mellitus. Gene Ther. 2016;23(4):323.
Singh A, Bhatia E, Dabadghao P, Bhatia V, Gellert S, Colman P. Role of islet autoimmunity in the aetiology of different clinical subtypes of diabetes mellitus in young north Indians. Diabet Med. 2000;17(4):275–80.
Poodineh M, Saravani R, Mirhosseini M, Sargazi S. Association of two methylenetetrahydrofolate reductase polymorphisms (rs1801133, rs1801131) with the risk of type 2 diabetes in South-East of Iran. Rep Biochem Mol Biol. 2019;8(2):178.
Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Investig. 2000;106(4):473–81.
Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90(5):3–10.
Galavi H, Mollashahee-Kohkan F, Saravani R, Sargazi S, Noorzehi N, Shahraki H. HHEX gene polymorphisms and type 2 diabetes mellitus: A case‐control report from Iran. J Cell Biochem. 2019;120(10):16445–51.
Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622(1–2):84–93.
Smidt K, Rungby J. ZnT3: a zinc transporter active in several organs. Biometals. 2012;25(1):1–8.
Syring KE, Boortz KA, Oeser JK, Ustione A, Platt KA, Shadoan MK, et al. Combined deletion of Slc30a7 and Slc30a8 unmasks a critical role for ZnT8 in glucose-stimulated insulin secretion. Endocrinology. 2016;157(12):4534–41.
Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis. 2009;19(6):431–9.
Rungby J. Zinc, zinc transporters and diabetes. Diabetologia. 2010;53(8):1549–51.
Xiang J, Li X-Y, Xu M, Hong J, Huang Y, Tan J-R, et al. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. J Clin Endocrinol Metab. 2008;93(10):4107–12.
Zhao T, Huang Q, Su Y, Sun W, Huang Q, Wei W. Zinc and its regulators in pancreas. Inflammopharmacology. 2019:1–12.
Weijers RN. Three-dimensional structure of β-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol Metab Syndr. 2010;2(1):33.
Solomou A, Philippe E, Chabosseau P, Migrenne-Li S, Gaitan J, Lang J, et al. Over-expression of Slc30a8/ZnT8 selectively in the mouse α cell impairs glucagon release and responses to hypoglycemia. Nutr Metab. 2016;13(1):46.
Faghih H, Khatami S-R, Azarpira N, Foroughmand A-M. SLC30A8 gene polymorphism (rs13266634 C/T) and type 2 diabetes mellitus in south Iranian population. Mol Biol Rep. 2014;41(5):2709–15.
Consortium GP. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Wang X, Li W, Ma L, Ping F, Liu J, Wu X, et al. Investigation of miRNA-binding site variants and risk of gestational diabetes mellitus in Chinese pregnant women. Acta Diabetol. 2017;54(3):309–16.
Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):13–28.
Motavallian A, Andalib S, Vaseghi G, Mirmohammad-Sadeghi H, Amini M. Association between PRO12ALA polymorphism of the PPAR-γ2 gene and type 2 diabetes mellitus in Iranian patients. Indian J Hum Genet. 2013;19(2):239.
Hosseini SRA, Hejazi K. The effects of Ramadan fasting and physical activity on blood hematological-biochemical parameters. Iran J Basic Med Sci. 2013;16(7):845.
Asemani S, Montazeri V, Baradaran B, Tabatabiefar MA, Pirouzpanah S. The effects of Berberis vulgaris juice on insulin indices in women with benign breast disease: a randomized controlled clinical trial. Iran J Pharm Res. 2018;17(Suppl):110.
Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215.
Collins A, Ke X. Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinforma J. 2012;6(1).
Ye S, Humphries S, Green F. Allele specific amplification by tetra-primer PCR. Nucleic Acids Res. 1992;20(5):1152.
Sabarinathan R, Tafer H, Seemann SE, Hofacker IL, Stadler PF, Gorodkin J. The RNAsnp web server: predicting SNP effects on local RNA secondary structure. Nucleic Acids Res. 2013;41(W1):W475–9.
Hashemi M, Bahari G, Sarhadi S, Eskandari E, Narouie B, Taheri M, et al. 4-bp insertion/deletion (rs3783553) polymorphism within the 3′ UTR of IL1A contributes to the risk of prostate cancer in a sample of Iranian population. J Cell Biochem. 2018;119(3):2627–35.
Desmet F-O, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67-e.
Liu C, Zhang F, Li T, Lu M, Wang L, Yue W, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;13(1):661.
Yong Y, Lin H. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15(2):97.
Jing Y, Sun Q, Bi Y, Shen S, Zhu D. SLC30A8 polymorphism and type 2 diabetes risk: evidence from 27 study groups. Nutr Metab Cardiovasc Dis. 2011;21(6):398–405.
Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, et al. A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes. 2008;57(4):1043–7.
Goda N, Murase H, Kasezawa N, Goda T, Yamakawa-Kobayashi K. Polymorphism in microRNA-binding site in HNF1B influences the susceptibility of type 2 diabetes mellitus: a population based case–control study. BMC Med Genet. 2015;16(1):75.
Gomes KFB, Semzezem C, Batista R, Fukui RT, Santos AS, Correia MR, et al. Importance of zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in Latin Americans. Sci Rep. 2017;7(1):1–7.
Kulkarni H, Mamtani M, Peralta JM, Diego V, Dyer TD, Goring H, et al. Lack of association between SLC30A8 variants and type 2 diabetes in Mexican American families. J Diabetes Res. 2016;2016.
Kirchhoff K, Machicao F, Haupt A, Schäfer S, Tschritter O, Staiger H, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51(4):597–601.
Mitchell RK, Hu M, Chabosseau PL, Cane MC, Meur G, Bellomo EA, et al. Molecular genetic regulation of Slc30a8/ZnT8 reveals a positive association with glucose tolerance. Mol Endocrinol. 2016;30(1):77–91.
Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a β-cell–specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53(9):2330–7.
Wijesekara N, Dai F, Hardy A, Giglou P, Bhattacharjee A, Koshkin V, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–68.
Tabara Y, Osawa H, Kawamoto R, Onuma H, Shimizu I, Miki T, et al. Replication study of candidate genes associated with type 2 diabetes based on genome-wide screening. Diabetes. 2009;58(2):493–8.
Rong R, Hanson RL, Ortiz D, Wiedrich C, Kobes S, Knowler WC, et al. Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes. 2009;58(2):478–88.
Cauchi S, Meyre D, Durand E, Proença C, Marre M, Hadjadj S, et al. Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PloS One. 2008;3(5):e2031.
Mohaddes S, Karami F, Gharesouran J, Bahrami A. The soluble carrier 30 A8 (SLC30A8) gene polymorphism and risk of Diabetes Mellitus Type 2 in Eastern Azerbijan population of Iran. J Sci, Islamic Republic of Iran. 2012;23(1):15–20.
DeMenna J, Puppala S, Chittoor G, Schneider J, Kim JY, Shaibi GQ, et al. Association of common genetic variants with diabetes and metabolic syndrome related traits in the Arizona Insulin Resistance registry: a focus on Mexican American families in the Southwest. Hum Hered. 2014;78(1):47–58.
Cheng L, Zhang D, Zhou L, Zhao J, Chen B. Association between SLC30A8 rs13266634 polymorphism and type 2 diabetes risk: a meta-analysis. Med Sci Monit. 2015;21:2178.
Cauchi S, Del Guerra S, Choquet H, D’Aleo V, Groves CJ, Lupi R, et al. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100(1):77–82.
Acknowledgements
We are grateful to Dr. Maryam Piri and Dr. Hamed Taheri, the two diabetes specialist physicians, for their assistance. We also wish to thank all the individuals who voluntarily participated in this study.
Funding
This work was financially supported by Zahedan University of Medical Sciences (Grant No. 8256).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sargazi, S., Heidari Nia, M., Sargazi, F.M. et al. SNPs in the 3′-untranslated region of SLC30A8 confer risk of type 2 diabetes mellitus in a south-east Iranian population: Evidences from case-control and bioinformatics studies. J Diabetes Metab Disord 19, 979–988 (2020). https://doi.org/10.1007/s40200-020-00590-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00590-5