Abstract
Purpose
Diabetes and its complications such as diabetic cardiomyopathy still account for significant morbidity and mortality. High-quality evidence was shown the importance of exercise in controlling diabetes complications, but the molecular mechanism on diabetic cardiomyopathy is not yet fully understood. This study aimed to compare and investigate the effect of high intensity interval training (HIIT) and continuous endurance training (CET) on the signaling pathway of diabetic cardiomyopathy.
Methods
Hence, 21 Wistar rats with an average weight of 260 ± 10 g, after induction of diabetes (STZ 50 mg/kg BW) were randomly divided into three groups (control, CET and HIIT; n = 7). Training programs were conducted 5 days a week for 5 weeks. CET program was defined as running at 60% vVO2max for 30 min in each session and the HIIT program was defined as running at 85–90% vVO2max for 3 min followed by 1 min recovery (30–35% vVO2max), that was repeated four times in each session. The cardiac performance was analyzed via determination of end systolic and diastolic dimensions and the ejection fraction by echocardiography. To elucidate the responsible molecular mechanism of miR-1, IGF-1 and IGF-1R mRNA and apoptosis marker protein expression were investigated.
Results
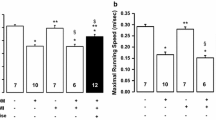
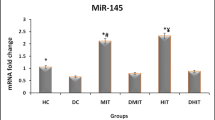
Both training programs specifically HIIT, significantly reduced the blood glucose, enhanced heart performance, reduced miR-1 expression, induced IGF-1 and IGF-1R expression and reduced apoptotic protein expression.
Conclusion
We showed that HIIT is more effective than CET for reduction of diabetic cardiomyopathy as a complication of diabetes in animal models through suppressing miR-1 and its downstream apoptosis pathway.



Similar content being viewed by others
References
Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Failure Rev. 2012;17:325–44.
Skyler JS. Diabetic complications: the importance of glucose control. Endocrinol Metab Clin N Am. 1996;25:243–54.
Khodabandeloo H, Gorgani-Firuzjaee S, Panahi S, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and beta cell dysfunction. Transl Res. 2016;167:228–56.
Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–9.
Bugger H. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci. 2008;114:195–210.
Yu X-Y, Song Y-H, Geng Y-J, Lin Q-X, Shan Z-X, Lin S-G, et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376:548–52.
Kuo W-W, Wang W-J, Tsai C-Y, Way C-L, Hsu H-H, Chen L-M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int J Cardiol. 2013;168:270–80.
Shan Z-X, Lin Q-X, Deng C-Y, Zhu J-N, Mai L-P, Liu J-L, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–600.
Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med. 2013;65:1435–46.
Mazloom H, Alizadeh S, Pasalar P, Esfahani EN, Meshkani R. Downregulated microRNA-155 expression in peripheral blood mononuclear cells of type 2 diabetic patients is not correlated with increased inflammatory cytokine production. Cytokine. 2015;76:403–8.
Van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–76.
Townley-Tilson WD, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42:1252–5.
Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S, Khakdan S. High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 2018:1–8.
Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120:2377–85.
Ghareghani P, Shanaki M, Ahmadi S, Khoshdel AR, Rezvan N, Meshkani R, et al. Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes Res Clin Pract. 2018;12:80–9.
Shantikumar S, Caporali A, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93:583–93.
Yu X-Y, Geng Y-J, Liang J-L, Lin Q-X, Lin S-G, Zhang S, et al. High levels of glucose induce apoptosis in cardiomyocyte via epigenetic regulation of the insulin-like growth factor receptor. Exp Cell Res. 2010;316:2903–9.
Cheng S-M, Ho T-J, Yang A-L, Chen I-J, Kao C-L, Wu F-N, et al. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. Int J Cardiol. 2013;167:478–85.
Miller Y, Dunstan D. The effectiveness of physical activity interventions for the treatment of overweight and obesity and type 2 diabetes. J Sci Med Sport. 2004;7:52–9.
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33:2692–6.
Buchan DS, Ollis S, Young JD, Thomas NE, Cooper SM, Tong TK, et al. The effects of time and intensity of exercise on novel and established markers of CVD in adolescent youth. Am J Hum Biol. 2011;23:517–26.
Meyer P, Normandin E, Gayda M, Billon G, Guiraud T, Bosquet L, et al. High-intensity interval exercise in chronic heart failure: protocol optimization. J Card Fail. 2012;18:126–33.
Khakdan S, Delfan M, Heydarpour Meymeh M, Kazerouni F, Ghaedi H, Shanaki M, et al. High-intensity interval training (HIIT) effectively enhances heart function via miR-195 dependent cardiomyopathy reduction in high-fat high-fructose diet-induced diabetic rats. Arch Physiol Biochem. 2018:1–8.
Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111:1554–60.
Gorgani-Firuzjaee S, Khatami S, Meshkani R. SH2 domain-containing inositol 5-phosphatase (SHIP2) regulates de-novo lipogenesis and secretion of apoB100 containing lipoproteins in HepG2 cells. Biochem Biophys Res Commun. 2015;464:1028–33.
Epp RA, Susser SE, Morissette MP, Kehler DS, Jassal DS, Duhamel TA. Exercise training prevents the development of cardiac dysfunction in the low-dose streptozotocin diabetic rats fed a high-fat diet 1. Can J Physiol Pharmacol. 2012;91:80–9.
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–91.
Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Therapu. 2007;114:307–17.
Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium mitochondrial cytochrome c–mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–48.
Ho FM, Liu SH, Liau CS, Huang J, Lin-Shiau SY. High glucose–induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH2-terminal kinase and caspase-3. Circulation. 2000;101:2618–24.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, et al. (2017) ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2017;70(6):776–803.
Acknowledgments
This work was financially supported by grants from Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (1392-02-97-1677). We thank Nandini Nair, Professor at Texas Tech University for her technical contribution and assist.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Delfan, M., Delphan, M., Kordi, M.R. et al. High intensity interval training improves diabetic cardiomyopathy via miR-1 dependent suppression of cardiomyocyte apoptosis in diabetic rats. J Diabetes Metab Disord 19, 145–152 (2020). https://doi.org/10.1007/s40200-019-00485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-019-00485-0