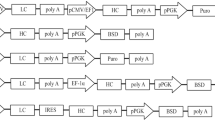
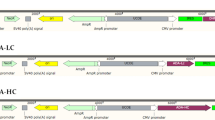
Abstract
Background
Producing therapeutic proteins can be done quickly and on a large scale through Transient Gene Expression (TGE). Chinese hamster ovary (CHO) cell lines are commonly used to achieve this. Although there are few comparative studies, TGE has been observed in suspension-adapted CHO cells.
Objectives
We tested TGE’s effectiveness in DG-44, CHO-S, and ExpiCHO-S cell lines with four transfection reagents.
Methods
A design of experiments (DoE) was followed to optimize transfection using a recombinant monoclonal antibody (mAb) construct. To evaluate the efficacy, flow cytometry and ELISA were used. Feeding strategies and temperature shifts were implemented to enhance transfection effectiveness. The quality of the mAb was assessed through ELISA, SDS-PAGE, and proliferation inhibition assays.
Results
We adapted all cell lines to grow in suspension using a serum-free medium. Our findings from flow cytometry and ELISA tests indicate that PEI and Pmax reagents had a higher rate of transfection and mAb production than the ExpiCHO commercial transfection reagent. While DG-44 cells had better transfection efficiency than CHO-S and ExpiCHO-S, there was no significant difference between CHO-S and ExpiCHO-S. Our TGE system was more productive at 32 °C than at 37 °C. In the optimized TGE of Pmax-based transfection in DG-44 at 37 and 32 °C, the production level of mAb was more than half of the amount of the commercial ExpiCHO-S expression system. Still, the number of transfected cells was three times higher, making it more efficient. The purified mAb from all transfected cell lines had similar structural and functional properties under different conditions.
Conclusion
Our research shows that using Pmax and DG-44 cells in the TGE system is a cost-effective and efficient way to produce humanized monoclonal antibodies. We discovered that this method outperforms the ExpiCHO-S kit.
Graphical abstract








Similar content being viewed by others
Data availability
All raw data were uploaded as Supplementary files.
References
Pham PL, Kamen A, Durocher Y. Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol. 2006;34(2):225–37. https://doi.org/10.1385/mb:34:2:225.
Geisse S. Reflections on more than 10 years of TGE approaches. Protein Exp Purif. 2008;64(2):99–107. https://doi.org/10.1016/j.pep.2008.10.017.
Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett. 2007;29(5):677–84. https://doi.org/10.1007/s10529-006-9297-y.
Zhu J. Update on production of recombinant therapeutic protein: transient gene expression. 1st ed. iSmithers Rapra Publishing; 2013.
Bollin F, Dechavanne V, Chevalet L. Design of experiment in CHO and HEK transient transfection condition optimization. Protein Exp Purif. 2011;78(1):61–8.
Ye J, Kober V, Tellers M, Naji Z, Salmon P, Markusen JF. High-level protein expression in scalable CHO transient transfection. Biotechnol Bioeng. 2009;103(3):542–51. https://doi.org/10.1002/bit.22265.
Rajendra Y, Hougland MD, Alam R, Morehead TA, Barnard GC. A high cell density transient transfection system for therapeutic protein expression based on a CHO GS-knockout cell line: process development and product quality assessment. Biotechnol Bioeng. 2015;112(5):977–86. https://doi.org/10.1002/bit.25514.
Gutiérrez-Granados S, Cervera L, Kamen AA, Gòdia F. Advancements in mammalian cell transient gene expression (TGE) technology for accelerated production of biologics. Crit Rev Biotechnol. 2018;38(6):918–40. https://doi.org/10.1080/07388551.2017.1419459.
Stuible M, Burlacu A, Perret S, Brochu D, Paul-Roc B, Baardsnes J, Loignon M, Grazzini E, Durocher Y. Optimization of a high-cell-density polyethylenimine transfection method for rapid protein production in CHO-EBNA1 cells. J Biotechnol. 2018;281:39–47. https://doi.org/10.1016/j.jbiotec.2018.06.307.
Derouazi M, Martinet D, Schmutz NB, Flaction R, Wicht M, Bertschinger M, Hacker D, Beckmann J, Wurm F. Genetic characterization of CHO production host DG44 and derivative recombinant cell lines. Biochem Biophys Res Commun. 2006;340(4):1069–77. https://doi.org/10.1016/j.bbrc.2005.12.111.
Barron N, Kumar N, Sanchez N, Doolan P, Clarke C, Meleady P, O’sullivan F, Clynes M. Engineering CHO cell growth and recombinant protein productivity by overexpression of miR-7. J Biotechnol. 2011;151(2):204–11. https://doi.org/10.1016/j.jbiotec.2010.12.005.
You M, Liu Y, Chen Y, Guo J, Wu J, Fu Y, Shen R, Qi R, Luo W, et al. Maximizing antibody production in suspension-cultured mammalian cells by the customized transient gene expression method. Biosci Biotechnol Biochem. 2013;77(6):1207–13. https://doi.org/10.1271/bbb.120968.
Longo PA, Kavran JM, Kim M-S, Leahy DJ. Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol. 2013;529:227–40. https://doi.org/10.1016/b978-0-12-418687-3.00018-5.
González-Domínguez I, Grimaldi N, Cervera L, Ventosa N, Gòdia F. Impact of physicochemical properties of DNA/PEI complexes on transient transfection of mammalian cells. New Biotechnol. 2019;49:88–97. https://doi.org/10.1016/j.nbt.2018.09.005.
Derouazi M, Girard P, Van Tilborgh F, Iglesias K, Muller N, Bertschinger M, Wurm FM. Serum-free large‐scale transient transfection of CHO cells. Biotechnol Bioeng. 2004;87(4):537–45. https://doi.org/10.1002/bit.20161.
Sellhorn G, Caldwell Z, Mineart C, Stamatatos L. Improving the expression of recombinant soluble HIV envelope glycoproteins using pseudo-stable transient transfection. Vaccine. 2009;28(2):430–6. https://doi.org/10.1016/j.vaccine.2009.10.028.
Delafosse L, Xu P, Durocher Y. Comparative study of polyethylenimines for transient gene expression in mammalian HEK293 and CHO cells. J Biotechnol. 2016;227:103–11. https://doi.org/10.1016/j.jbiotec.2016.04.028.
Nair RR, Rodgers JR, Schwarz LA. Enhancement of transgene expression by combining glucocorticoids and anti-mitotic agents during transient transfection using DNA-cationic liposomes. Mol Ther. 2002;5(4):455–62. https://doi.org/10.1006/mthe.2002.0567.
Legendre J-Y, Szoka FC Jr. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes. Pharm Res. 1992;9(10):1235–42. https://doi.org/10.1023/a:1015836829670.
Zhong X, Ma W, Meade CL, Tam AS, Llewellyn E, Cornell R, Cote K, Scarcelli JJ, Marshall JK, et al. Transient CHO expression platform for robust antibody production and its enhanced N-glycan sialylation on therapeutic glycoproteins. Biotechnol Prog. 2019;35(1):e2724. https://doi.org/10.1002/btpr.2724.
Jain NK, Barkowski-Clark S, Altman R, Johnson K, Sun F, Zmuda J, Liu CY, Kita A, Schulz R, et al. A high density CHO-S transient transfection system: comparison of ExpiCHO and Expi293. Protein Expr Purif. 2017;134:38–46. https://doi.org/10.1016/j.pep.2017.03.018.
Liu CY, Spencer V, Kumar S, Liu J, Chiou H, Zmuda JF. Attaining high transient titers in CHO cells: case study involving the use of the ExpiCHO™ mammalian transient expression system. Genetic Eng Biotechnol News. 2015;35(17):34–5. https://doi.org/10.1089/gen.35.17.15.
Cupo A, Cruz Portillo VM, Gelfand P, Yasmeen A, Klasse P, Moore JP. Optimizing the production and affinity purification of HIV-1 envelope glycoprotein SOSIP trimers from transiently transfected CHO cells. PLoS ONE. 2019;14(4):e0215106.DOI. https://doi.org/10.1371/journal.pone.0215106.
Amiri MM, Golsaz-Shirazi F, Soltantoyeh T, Hosseini-Ghatar R, Bahadori T, Khoshnoodi J, Navabi SS, Farid S, Karimi-Jafari MH, et al. Hersintuzumab: a novel humanized anti-HER2 monoclonal antibody induces potent tumor growth inhibition. Investig New Drugs. 2018;36(2):171–86. https://doi.org/10.1007/s10637-017-0518-0.
Tahmasebi F, Kazemi T, Amiri MM, Khoshnoodi J, Mahmoudian J, Bayat AA, Jeddi-Tehrani M, Rabbani H, Shokri F. Vitro assessment of the effects of anti-HER2 monoclonal antibodies on proliferation of HER2-overexpressing breast cancer cells. Immunotherapy. 2014;6(1):43–9. https://doi.org/10.2217/imt.13.156.
Kazemi T, Tahmasebi F, Bayat AA, Mohajer N, Khoshnoodi J, Jeddi-Tehrani M, Rabbani H, Shokri F. Characterization of novel murine monoclonal antibodies directed against the extracellular domain of human HER2 tyrosine kinase receptor. Hybridoma. 2011;30(4):347–53. https://doi.org/10.1089/hyb.2011.0023.
Brezinsky SCG, Chiang GG, Szilvasi A, Mohan S, Shapiro RI, MacLean A, Sisk W, Thill G. A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods. 2003;277(1):141–55. https://doi.org/10.1016/S0022-1759(03)00108-X.
Borth N, Zeyda M, Katinger H. Efficient selection of high-producing subclones during gene amplification of recombinant chinese hamster ovary cells by flow cytometry and cell sorting. Biotechnol Bioeng. 2000;71(4):266–73. https://doi.org/10.1002/1097-0290(2000)71:4<266::AID-BIT1016>3.0.CO;2-2.
Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J Struct Funct Genomics. 2005;6(2):165–70. https://doi.org/10.1007/s10969-005-2826-4.
Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol. 1999;10(2):156–9. https://doi.org/10.1016/s0958-1669(99)80027-5.
You M, Yang Y, Zhong C, Chen F, Wang X, Jia T, Chen Y, Zhou B, Mi Q, et al. Efficient mAb production in CHO cells with optimized signal peptide, codon, and UTR. Appl Microbiol Biotechnol. 2018;102(14):5953–64. https://doi.org/10.1007/s00253-018-8986-5.
Sou SN, Lee K, Nayyar K, Polizzi KM, Sellick C, Kontoravdi C. Exploring cellular behavior under transient gene expression and its impact on mAb productivity and Fc-glycosylation. Biotechnol Bioeng. 2018;115(2):512–8. https://doi.org/10.1002/bit.26456.
Ye J, Alvin K, Latif H, Hsu A, Parikh V, Whitmer T, Tellers M, de la Edmonds MC, Ly J, et al. Rapid protein production using CHO stable transfection pools. Biotechnol Prog. 2010;26(5):1431–7. https://doi.org/10.1002/btpr.469.
Wright JL, Jordan M, Wurm FM. Extraction of plasmid DNA using reactor scale alkaline lysis and selective precipitation for scalable transient transfection. Cytotechnology. 2001;35(3):165–73. https://doi.org/10.1023/a:1013106032341.
Steger K, Brady J, Wang W, Duskin M, Donato K, Peshwa M. CHO-S antibody titers > 1 gram/liter using flow electroporation-mediated transient gene expression followed by rapid migration to high-yield stable cell lines. J Biomol Screen. 2015;20(4):545–51. https://doi.org/10.1177/1087057114563494.
Reinhart D, Damjanovic L, Sommeregger W, Gili A, Schafellner S, Castan A, Kaisermayer C, Kunert R. Influence of cell culture media and feed supplements on cell metabolism and quality of IgG produced in CHO-K1, CHO-S, and CHO-DG44. BMC Proc. 2015; 9(9):36. https://doi.org/10.1186/1753-6561-9-S9-P36.
Chiou HC, Vasu S, Liu CY, Cisneros I, Jones MB, Zmuda JF. Scalable transient protein expression. Methods Mol Biol. 2014;1104:35–55. https://doi.org/10.1007/978-1-62703-733-4_4.
Geisse S, Voedisch B. Transient expression technologies: past, present, and future. Methods Mol Biol. 2012;899:203–19. https://doi.org/10.1007/978-1-61779-921-1_13.
Wulhfard S, Tissot S, Bouchet S, Cevey J, De Jesus M, Hacker DL, Wurm FM. Mild hypothermia improves transient gene expression yields several fold in Chinese hamster ovary cells. Biotechnol Prog. 2008;24(2):458–65. https://doi.org/10.1021/bp070286c.
Funding
This study was partially supported by grants from National Institute for Medical Research Development of Iran (Grant No. 964157) and Tehran University of Medical Sciences (Grant No. 98-3-99-41359).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics statement
The animal study was reviewed and approved by the ethics committee of the National Institute for Medical Research Development of Iran - NIMAD (IR NIMAD REC 1396 060).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 225 KB)
ESM 2
(CSV 308 bytes)
ESM 3
(CSV 345 bytes)
ESM 4
(CSV 314 bytes)
ESM 5
(CSV 339 bytes)
ESM 6
(CSV 360 bytes)
ESM 7
(CSV 333 bytes)
ESM 8
(CSV 342 bytes)
ESM 9
(CSV 337 bytes)
ESM 10
(CSV 306 bytes)
ESM 11
(CSV 496 bytes)
ESM 12
(CSV 515 bytes)
ESM 13
(CSV 569 bytes)
ESM 14
(CSV 475 bytes)
ESM 15
(CSV 504 bytes)
ESM 16
(CSV 413 bytes)
ESM 17
(CSV 254 bytes)
ESM 18
(CSV 207 bytes)
ESM 19
(CSV 272 bytes)
ESM 20
(DOCX 406 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roshani, A., Mohammadi, M., Bahadori, T. et al. Comparison of different transient gene expression systems for the production of a new humanized anti-HER2 monoclonal antibody (Hersintuzumab). DARU J Pharm Sci 31, 221–231 (2023). https://doi.org/10.1007/s40199-023-00477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-023-00477-9