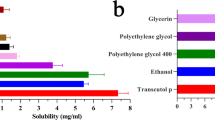
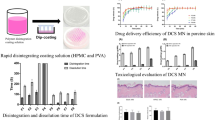
Abstract
Purpose
The aim of this study was to design and characterize microneedle patch formulation containing cetirizine hydrochloride.
Methods
Chitosan was co-formulated with cetirizine hydrochloride. Transdermal patches were prepared by casting this solution to microneedle molds. Control patches were formulated by casting this solution to a plain cuvet of same area as mold but lacking microneedles. An array of methods namely; differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM) were employed for the characterization of the films and the microneedles accordingly whereas in vitro permeation studies were conducted across rat skin. Light microscopy was performed to assess any histological changes upon microneedles application onto the rat skin.
Results
The patches had a reproducible thickness (0.86 ± 0.06 mm) and folding endurance. Both the blank and drug loaded patches had 100 microneedles each of 300 micrometre length. In addition, the microneedle patches were ascribed with a two-fold increase in drug permeation across rat skin in the presence of microneedles as compared to the control formulations. Histological examination confirms a minimal invasion of the skin conferred by the microneedles.
Conclusion
The microneedle patches serve as an alternate route of drug administration in patients with nausea and swelling difficulties.

Microneedle patch manifest a two-fold increase in the skin permeation of Cetirizine Hydrochloride as compared to the control that is drug loaded patch without microneedles







Similar content being viewed by others
References
Ranade VV, Hollinger MA. Drug delivery systems. FL, USA: CRC press Boca Raton; 2004.
Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technol To. 2000;3(9):318–26.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal drug delivery system: a review. The Pharma Innovation. 2012;1(4).
Rasekh M, Karavasili C, Soong YL, Bouropoulos N, Morris M, Armitage D, et al. Electrospun PVP–indomethacin constituents for transdermal dressings and drug delivery devices. Int J Pharm. 2014;473(1):95–104. https://doi.org/10.1016/j.ijpharm.2014.06.059.
Ahmad Z, Stride E, Edirisinghe M. Novel preparation of transdermal drug-delivery patches and functional wound healing materials. J Drug Target. 2009;17(9):724–9. https://doi.org/10.3109/10611860903085386.
Larrañeta E, Lutton RE, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mat Sci Eng: R: Reports. 2016;104:1–32.
Ita K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed Pharmacother. 2017;93:1116–27. https://doi.org/10.1016/j.biopha.2017.07.019.
Larrañeta E, McCrudden MT, Courtenay AJ, Donnelly RF. Microneedles: a new frontier in nanomedicine delivery. Pharm Res. 2016;33(5):1055–73.
Khan H, Mehta P, Msallam H, Armitage D, Ahmad Z. Smart microneedle coatings for controlled delivery and biomedical analysis. J Drug Target. 2014;22(9):790–5. https://doi.org/10.3109/1061186X.2014.921926.
Bystrova S, Luttge R. Micromolding for ceramic microneedle arrays. Microelectron Eng. 2011;88(8):1681–4.
Lee K, Jung H. Drawing lithography for microneedles: a review of fundamentals and biomedical applications. Biomaterials. 2012;33(30):7309–26.
Omatsu T, Chujo K, Miyamoto K, Okida M, Nakamura K, Aoki N, et al. Metal microneedle fabrication using twisted light with spin. Opt Express. 2010;18(17):17967–73. https://doi.org/10.1364/OE.18.017967.
Kim JD, Kim M, Yang H, Lee K, Jung H. Droplet-born air blowing: novel dissolving microneedle fabrication. J Control Release. 2013;170(3):430–6. https://doi.org/10.1016/j.jconrel.2013.05.026.
Park J-H, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–19.
Haj-Ahmad R, Khan H, Arshad M, Rasekh M, Hussain A, Walsh S, et al. Microneedle Coating Techniques for Transdermal Drug Delivery. Pharmaceutics. 2015;7(4):486.
Goindi S, Kumar G, Kumar N, Kaur A. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech. 2013;14(4):1284–93.
Wang M, Hu L, Xu C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip. 2017;17(8):1373–87.
Chen M-C, Ling M-H, Lai K-Y, Pramudityo E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules. 2012;13(12):4022–31.
Chang C, Wang Z-C, Quan C-Y, Cheng H, Cheng S-X, Zhang X-Z, et al. Fabrication of a novel pH-sensitive glutaraldehyde cross-linked pectin nanogel for drug delivery. J Biomater Sci Polym Ed. 2007;18(12):1591–9. https://doi.org/10.1163/156856207794761925.
Demir YK, Akan Z, Kerimoglu O. Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery. PLoS ONE. 2013;8(10):e77289. https://doi.org/10.1371/journal.pone.0077289.
Seok H-y, Suh H, Baek S, Kim Y-C. Microneedle applications for DNA vaccine delivery to the skin. DNA Vaccines: Methods and Protocols. 2014:141-58.
Ma Y, Gill HS. Coating Solid Dispersions on Microneedles via a Molten Dip-Coating Method: Development and In Vitro Evaluation for Transdermal Delivery of a Water-Insoluble Drug. J Pharm Sci. 2014;103(11):3621–30.
Moga KA, Bickford LR, Geil RD, Dunn SS, Pandya AA, Wang Y, et al. Rapidly–dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Adv Mater. 2013;25(36):5060–6.
Martin C, Allender CJ, Brain KR, Morrissey A, Birchall JC. Low temperature fabrication of biodegradable sugar glass microneedles for transdermal drug delivery applications. J Control Release. 2012;158(1):93–101.
Migalska K, Morrow DI, Garland MJ, Thakur R, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm Res. 2011;28(8):1919–30.
Gittard SD, Ovsianikov A, Akar H, Chichkov B, Monteiro-Riviere NA, Stafslien S et al. Two Photon Polymerization-Micromolding of Polyethylene Glycol-Gentamicin Sulfate Microneedles. Adv Eng Mate. 2010;12(4).
Auner BG, Valenta C, Hadgraft J. Influence of phloretin and 6-ketocholestanol on the skin permeation of sodium-fluorescein. J Control Release. 2003;89(2):321–8.
Xie Y, Xu B, Gao Y. Controlled transdermal delivery of model drug compounds by MEMS microneedle array. Nanomedicine. 2005;1(2):184–90.
Wankhede S, Lad K, Chitlange S. Development and validation of UV-spectrophotometric methods for simultaneous estimation of cetirizine hydrochloride and phenylephrine hydrochloride in tablets. Int J Pharm Sci Drug Res. 2012;4(3):222–6.
Bhatia NM, Ganbavale SK, More HN. Spectrophotometric estimation of ambroxol hydrochloride and cetirizine hydrochloride in tablets. Asian J Pharm. 2014;2(3).
van Essen HF, Verdaasdonk MA, Elshof SM, de Weger RA, van Diest PJ. Alcohol based tissue fixation as an alternative for formaldehyde: influence on immunohistochemistry. J Clin Pathol. 2010;63(12):1090–4. https://doi.org/10.1136/jcp.2010.079905.
Davis SP. Hollow microneedles for molecular transport across skin: Georgia Institute of Technology; 2004.
Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication. mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66.
Jana S, Trivedi M, Tallapragada RM, Branton A, Trivedi D, Nayak G, et al. Characterization of Physicochemical and Thermal Properties of Chitosan and Sodium Alginate after Biofield Treatment. Pharm Anal Acta. 2015;10(6).
Soares JP, Santos J, Chierice GO, Cavalheiro E. Thermal behavior of alginic acid and its sodium salt. Eclética Química. 2004;29(2):57–64.
Guinesi LS, Cavalheiro ETG. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim Acta. 2006;444(2):128–33.
Acknowledgements
The authors acknowledge the financial support provided by Higher Education Commision of Pakistan under National Research Program for Universities (NRPU) vide No: 7401/Punjab/NRPU/R&D/HEC/2017.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation of the manuscript and the study (i.e. through various streams be it planning, experiments, analysis of data, data preparation etc).
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arshad, M.S., Hassan, S., Hussain, A. et al. Improved transdermal delivery of cetirizine hydrochloride using polymeric microneedles. DARU J Pharm Sci 27, 673–681 (2019). https://doi.org/10.1007/s40199-019-00301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00301-3