Abstract
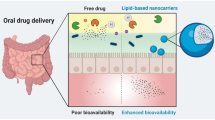
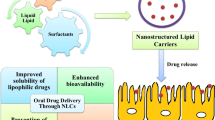
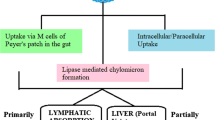
Hydrophilic drugs are preferred candidates for most routes of drug administration, because of their enhanced solubility and dissolution under aqueous in vivo conditions. However, their hydrophilic nature also leads to decreased permeability across hydrophobic barriers. This is a severe limitation in situations where membrane permeability is the primary factor affecting bioavailability and efficacy of the drug. Highly impermeable cellular membranes or the tight endothelial junctions governing the blood-brain barrier are prime examples of this limitation. In other cases, decreased permeability across mucosal or epithelial membranes may require increased doses, which is an inefficient and potentially dangerous workaround. Covalent conjugation of hydrophilic drugs to hydrophobic moieties like short-chain lipids is a promising strategy for maintaining the critical balance between drug solubility and permeability. This article practically focuses on the production procedure of Lipid drug conjugates (LDCs), various formulation methodologies for preparing LDC nanoparticles with detailed about their in vitro physicochemical characterization at laboratory scale. Moreover, brief overviews on the role of LDCs in novel drug delivery applications as a substrate to various disease therapies are provided.

Three dimensional (3-D) schematic representation of LDCs structures.




Similar content being viewed by others
Abbreviations
- LNFs:
-
Lipid Nanoparticle Formulations
- LDCs:
-
Lipid-drug Conjugates
- NPs:
-
Nanoparticles
- GIT:
-
Gastrointestinal Tract
- ZP:
-
Zeta Potential
- PDI:
-
Polydispersity Index
- BA:
-
Bioavailability
- BCS:
-
Biopharmaceutical Classification Systems
References
Anthony AA, Mumuni AM, Philip FB. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. Recent Advances in Novel Drug Carrier Systems. Intech Open; 2012. Pp. 107–140.
Morel S, Terreno E, Ugazio E, Aime S, Gasco MR. NMR relaxometric investigations of lipid nanoparticles (SLN) containining gadolinium (III) complexes. Eur J Pharm Biopharm. 1998;45(2):157–63.
Muchow M, Maincent P, Müller RH. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev Ind Pharm. 2008;34(12):1394–405.
Olbrich C, Gessner A, Kayser O, Mueller RH. Lipid drug conjugate nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazene aceturate. J Drug Target. 2002;10(5):387–96.
Das RJ, Baishya K, Pathak K. Recent advancement of lipid drug conjugate as nanoparticulate drug delivery system. Int Res J Pharm. 2013;4(1):73–8.
Nakajima N, Ikada Y. Mechanism of amide formation by carbodiimides for bioconjugation in aqueous media. Bioconjug Chem. 1995;6(1):123–30.
Pignatello R, Spampinato G, Sorrenti V, Di Giacomo C, Vicari L, McGuire JJ, et al. Lipophilic methotrexate conjugates with antitumor activity. Eur J Pharm Sci. 2000;10(3):237–45.
Sharma P, Dube B, Sawant K. Synthesis of Cytarabine lipid drug conjugate for treatment of meningeal leukemia: development, characterization and In vitro cell line studies. J Biomed Nanotechnol. 2012;8(6):928–37.
Scriba GK. Phenytoin-lipid conjugates as potential prodrugs of phenytoin. Arch Pharm. 1993;326(8):477–81.
Neupane YR, Sabir MD, Ahmad N, Ali M, Kohli K. Lipid drug conjugate nanoparticle as a novel lipid nanocarrier for the oral delivery of decitabine: Ex-vivo gut permeation studies. Nanotechnology. 2013;24(41):1–11.
Olbrich C, Gessner A, Schröder W, Kayser O, Müller RH. Lipid drug conjugate nanoparticles of the hydrophilic drug diminazene-cytotoxicity testing and mouse serum adsorption. J Control Release. 2004;96(3):425–35.
Banerjee S, Pillai J. Lipid Nanoparticle Formulations for Enhanced Anti-tuberculosis Therapy. Holban AM, Grumezescu AM. Nanoarchitectonics for Smart Delivery and Drug Targeting. United Kingdom: Elsevier; 2016. Pp 285–313.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76.
Banerjee S, Chattopadhyay P, Ghosh A, Goyary D, Karmakar S, Veer V. Influence of process variables on essential oil microcapsule properties by carbohydrate polymer-protein blends. Carbohydr Polym. 2013;93(2):691–7.
Wen B, Sun Y, Xu Y, Sun J, Liu X, Wang Y, et al. Pharmacokinetic characteristics of the cytarabine prodrug, ilecytarabine, after intravenous and oral administration to rats. Asian J Pharm Sci. 2008;3:200.
Knothe G, Kenar JA. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur J Lipid Sci Technol. 2004;106:88–96.
Ferreira L, Vidal MM, Gil MH. Evaluation of poly(2- hydroxyethyl methacrylate) gels as drug delivery systems at different pH value. Int J Pharm. 2000;194(2):169–80.
Ren S, Yang S, Zhao Y, Yu T, Xiao X. Preparation and characterization of an ultrahydrophobic surface based on a stearic acid self-assembled monolayer over polyethyleneimine thin films. Surf Sci. 2003;546(2–3):64–74.
Charman WN, Stella VJ, editors. Lymphatic transport of drugs. Boca Raton: CRC Press; 1992.
Müller RH, Runge SA, Ravelli V, Thünemann AF, Mehnert W, Souto EB. Cyclosporine-loaded solid lipid nanoparticles (SLN®):drug-lipid physicochemical interactions and characterization of drug incorporation. Eur J Pharm Biopharm. 2008;68(3):535–44.
Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–9.
Liu D, Liu C, Weiwei Z, Zhang N. Enhanced gastrointestinal absorption of N-3-O-toluyl-fluorouracil by cationic solid lipid nanoparticles. J Nanopart Res. 2010;12(3):975–84.
Zhang J, Fan Y, Smith E. Experimental design for the optimization of lipid nanoparticles. J Pharm Sci. 2009;98(5):1813–9.
Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates-a review. J Control Release. 2008;128(3):185–99.
Xie S, et al. Formulation, characterization, and pharmacokinetics of praziquantel- loaded hydrogenated castor oil solid lipid nanoparticles. Nanomedicine London. 2010;5(5):693–701.
Sanjula B, Shah FM, Javed A, Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17(3):249–56.
Paliwal R, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine. 2009;5(2):184–91.
Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN) dispersions. Int J Pharm. 1998;168(2):221–9.
Radomska-Soukharev A. Stability of lipid excipients in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):411–8.
Mukherjee B, Santra K, Pattnaik G, Ghosh S. Preparation, characterization and in vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int J Nanomedicine. 2008;3(4):487–96.
Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, et al. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100(2):547–57.
Varshosaz J, Minayian M, Moazen E. Enhancement of oral bioavailability of pentoxifylline by solid lipid nanoparticles. J Liposome Res. 2010;20(2):115–23.
Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2010;55(3):495–503.
Sahana B, Santra K, Basu S, Mukherjee B. Development of biodegradable polymer-based tamoxifen citrate-loaded nanoparticles and effect of some manufacturing process parameters on them: a physicochemical and in vitro evaluation. Int J Nanomedicine. 2010;5(7):621–30.
Shahgaldian P, Da Silva E, Coleman AW, Rather B, Zaworotko MJ. Para-acyl-calixarene based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Int J Pharm. 2003;253(1–2):23–38.
Schwarz C, Freitas C, Mehnert CW, Muller RH. Sterilization and physical stability of drug-free and etomidate- loaded solid lipid nanoparticles. Proc Int Symp Control Release Bioact Mater. 1995;22:766–7.
Zur Muhlen A, et al. Atomic force microscopy studies of solid lipid nanoparticles. Pharm Res. 1996;13(9):1411–6.
Jenning V, Thunemann A, Gohla S. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167–77.
Sari A, Akcay M, Soylak M, Onal A. Polymer-stearic acid blends as form-stable phase change material for thermal energy storage. J Sci Ind Res. 2005;64:991–6.
Dicko A, et al. Biophysical characterization of a liposomal formulation of cytarabine and daunorubicin. Int J Pharm. 2010;391(1–2):248–59.
Souto EB, Mehnert W, Muller RH. Polymorphic behavior of Comprito l888 ATO as bulk lipid and as SLN and NLC. J Microencapsul. 2006;23(4):417–33.
Bunjes H, Steiniger F, Richter W. Visualizing the structure of triglyceride nanoparticles in different crystal modifications. Langmuir. 2007;23(7):4005–11.
Estella-Hermoso de Mendoza A, Rayo M, Mollinedo M, Blanco-Prieto MJ. Lipid nanoparticles for alkyl lysophospholipid edel fosine encapsulation: development and in vitro characterization. Eur J Pharm Biopharm. 2008;68(2):207–13.
Huang ZR, Hua SC, Yang YL, Fang JY. Development and evaluation of lipid nanoparticles for camptothecin delivery: a comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol Sin. 2008;29(9):1094–102.
Banerjee S, Roy S, Nath Bhaumik K, Kshetrapal P, Pillai J. Comparative study of oral lipid nanoparticle formulations (LNFs) for chemical stabilization of antitubercular drugs: physicochemical and cellular evaluation. Artif Cells Nanomed Biotechnol. 2018;26:1–19.
Stela G, Esther I. Conjugates for cancer therapy and diagnosis, patent application number: 20110275590; Publication date: 11, October (2011).
Wyatt DA. Taking poorly water-soluble compounds through discovery. In: Recent advances in the formulations and development of poorly soluble drugs. Bulletin Technique Gattefosse. 1999:31–39.
Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–5.
Penzesa CB, Schnoller D, Horvati K, Kiss E. Membrane affinity of antituberculosis drug conjugate using lipid monolayer containing mycolic acid. Colloids Surf A Physicochem Eng Asp. 2012;413(5):142–8.
Vadlapudia AD, Vadlapatla RK, Kwatra D, Earla R, Samanta SK. Targeted lipid-based drug conjugates: a novel strategy for drug delivery. Int J Pharm. 2012;434(1–2):315–24.
Paliwal R, Shivani RP, Govind PA, Suresh PV. Biomimetic solid lipid nanoparticles for oral bioavailability enhancement of low molecular weight heparin and its lipid conjugates: In vitro and in-vivo evaluation. Mol Pharm. 2011;8(4):1314–21.
Sarpietro MG, Ottimo S, Giuffrida MC, Rocco F, Ceruti M, Castelli F. Synthesis of n-squalenoyl cytarabine and evaluation of its affinity with phospholipid bilayers and monolayers. Int J Pharm. 2011;406(1–2):69–77.
Ali SM, Khan AR, Ahmad MU, Chen P, Sheikh S, Ahmad I. Synthesis and biological evaluation of gemcitabine-lipid conjugate. Bioorg Med Chem Lett. 2005;15(10):2571–4.
Gessner A, Olbrich C, Schroder W, Kayser O, Muller RH. The role of plasma proteins in brain targeting: species-dependent protein adsorption patterns on brain-specific lipid drug conjugate (LDC) nanoparticles. Int J Pharm. 2001;214(1–2):87–91.
Kurz M, Scriba GK. Drug-phospholipid conjugates as potential prodrugs: synthesis, characterization, and degradation pancreatic phospholipase A2. Chem Phys Lipids. 2000;107(2):143–57.
Sugarman SM, Zou Y, Wasan K, Poirot K, Kumi R, Reddy S, et al. Lipid-complexed camptothecin: formulation and initial biodistribution and antitumor activity studies. Cancer Chemother Pharmacol. 1996;37(6):531–8.
Toth I, Hughes RA, Dekany G, Hillery AM, Ward P. Synthesis and oral uptake studies of lipidic and glyco-lipidic conjugates of β- lactam antibiotics. Liebigs Annalen Der Chemie. 1994;1994(7):685–8.
Lambert DM. Rationale and applications of lipids as prodrug carriers. Eur J Pharm Sci. 2000;11(2):S15–27.
Acknowledgments
The authors are thankful to their respective institution and university for providing access to necessary literature resources and essential library facilities for writing this review article. Recognition also goes to all the authors of papers, books, patents, websites and all other published sources listed in the references that were used to prepare the contents of this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Declaration of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Banerjee, S., Kundu, A. Lipid-drug conjugates: a potential nanocarrier system for oral drug delivery applications. DARU J Pharm Sci 26, 65–75 (2018). https://doi.org/10.1007/s40199-018-0209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-018-0209-1