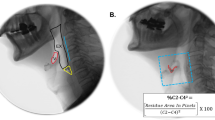
Abstract
Purpose of Review
Difficulty swallowing (dysphagia) is of great concern to patients with ALS as its complications can increase mortality and reduce the quality of life. This review aims to provide an overview of the recent developments and the current state of assessment, treatment, and management of dysphagia in ALS.
Recent Findings
The optimal timing of assessment, treatment, and management of dysphagia may be early in the ALS disease process, even before the dysphagia occurs. There is wide heterogeneity in SLP practice patterns for the management of dysphagia.
Summary
Dysphagia is common and debilitating; however, for various reasons, there is no clear consensus on how best to manage dysphagia in this population. Future work centered around predicting swallowing decline and improving interventions aimed at prolonging swallowing function in the early stages of the disease process may promote improved dysphagia care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 5 years, research has markedly advanced our understanding of the assessment and treatment of devastating swallowing impairments that impact most individuals living with amyotrophic lateral sclerosis (ALS) [1]. While the onset of dysphagia typically occurs sooner for those whose symptoms begin in the bulbar regions, the timing of dysphagia development and progression varies widely among individuals. Dysphagia is of great concern to those affected by ALS as its complications, such as aspiration pneumonia, have been found to increase mortality [2] and reduce the quality of life [3].
This review aims to provide an overview of ALS-related dysphagia consisting of the current state of healthcare practice, the timing of assessments and interventions, the development and validation of examinations, the effectiveness of exercises, and the benefits of oral and non-oral nutritional management methods. It begins with a brief background of ALS-related dysphagia, followed by discussions of (1) commonly used and new assessment methodologies, (2) rehabilitative and compensatory interventions, and (3) recommendations for future research. Table 1 summarizes the studies dated from 2017 to 2022 used in this review, categorized by the levels of evidence classification system described by Dang et al. [36].
Swallowing Function in ALS
Studies estimate that up to 92% of individuals with ALS experience dysphagia [1, 17, 37]. ALS-related dysphagia can result in physical complications, including aspiration pneumonia [2]; malnutrition and dehydration are predictors of survival in this population [38, 39]. Dysphagia can negatively impact the quality of life, including sleep quality, mental health, social interactions, and eating desire [40]. Additionally, it can increase general fatigue, eating duration, fear of eating-related complications, eating-related burden, and difficulty with food selection [3].
Much of the literature has focused on the oropharyngeal physiology of dysphagia. Signs and symptoms may differ depending on the onset type of ALS; individuals with bulbar onset often have earlier and more difficulty swallowing than those with spinal onset. Oral phase impairments include reductions in tongue speed, coordination, and range of motion; reduced tongue base retraction; reduced bolus preparation; the presence of oral bolus holding behavior; increased oral transit time; impaired anterior-posterior bolus transport; increased oral residue; and delayed initiation of the pharyngeal phase [2, 18, 19, 20, 41]. In the pharyngeal phase, common impairments include reduced anterior hyoid excursion; reduced laryngeal elevation; reduced pharyngeal constriction; increased vallecular and pyriform sinus residue amount; and increased number of aspiration and penetration events on liquids [2, 18, 41]. However, upper esophageal sphincter opening generally appears intact, even in the later stages of ALS [2].
Respiratory and swallowing functions in ALS are closely related, and weakness in the respiratory muscles can impair aspiration-protective mechanisms like coughing [31, 42]. Studies have found that among individuals with ALS, participants considered unsafe swallowers had reduced voluntary and reflexive cough effectiveness compared to safe swallowers, placing them at high risk for aspiration-related complications, including pneumonia [26, 42].
Assessment
Assessing swallowing through screens or evaluations is the first step toward developing a patient-specific care plan. Screens are quicker and easier to administer but often need more detailed information to make a treatment plan. Because of their simplicity, their primary purpose is to indicate if there is a need for further evaluation [15, 30, 43]. Evaluations are typically more time and resource extensive than screens. An evaluation aims to determine the presence and severity of dysphagia so that clinicians can develop comprehensive management or treatment plans. Evaluations may use easily accessible clinical materials or specialized instrumental assessments like videofluoroscopy (VFSS) or fiberoptic endoscopy (FEES). Instrumental assessments can determine (1) physiologic impairments present during swallowing, (2) consistencies safest for oral intake, and (3) compensatory strategies that best minimize aspiration and maximize swallow efficiency [15, 43]. Additionally, healthcare teams can use them to educate individuals and their caregivers about their swallowing impairment.
Current literature suggests that the indications and methods for and timing of swallowing assessments vary widely among providers and healthcare systems, resulting in inconsistent care within and between patients. Below, we summarize widely used swallowing screens and evaluations for individuals with ALS. Table 2 summarizes the psychometric properties of the tools included in this review. Note that some assessment tools need validation in the ALS population; thus, their sensitivity and specificity will not be available. In addition to swallowing, individuals with ALS have other bulbar impairments of salivation and speech. Yunusova et al. [30] provide a comprehensive overview of bulbar-related assessments in individuals with ALS.
Screens
The 3-oz water test and the Eating Assessment Tool-10 (EAT-10) are two commonly used screening tools that recently underwent validation studies in individuals with ALS. Both screens are quick and easy to administer, but the 3-oz water test is performance-based, whereas the EAT-10 is patient-reported. While task performance-based measures may be more objective, patient-reported outcomes may be more clinically meaningful.
Two studies have compared the 3-oz water test’s ability to detect aspiration or penetration to the penetration-aspiration scale (PAS) during VFSS. In a study with 31 participants with motor neuron disease including ALS (Focht), Garand et al. [15] found the screen detected aspiration with a sensitivity of 80% and a specificity of 33%. However, in their study with 212 participants with ALS, Donohue et al. [44] found that the 3-oz water test detected aspiration with a sensitivity of only 55% and a specificity of 72%. Based on their respective findings (Focht), Garand et al. recommend evaluation via VFSS if the individual does not pass the 3-oz water test, whereas Donohue et al. recommend against using it to screen for aspiration.
The EAT-10 is a self-reported questionnaire that probes the patient’s perspectives about their degree of swallowing impairment; the higher the score, the more severe the patient perceives their swallowing impairments to be [46]. In a group of 70 participants with ALS, Plowman et al. [43] compared the EAT-10 to the PAS during VFSS. They found that an EAT-10 score of 3 detects penetration and aspiration with a sensitivity of 88% and a specificity of 57%. Additionally, a score of 8 detects aspiration with a sensitivity of 86% and specificity of 72%. A later study by Donohue et al. [13] compared EAT-10 to the Dynamic Imaging Grade of Swallowing Toxicity scale in 273 participants with ALS. They found that an EAT-10 score of 3 detects mild dysphagia with a sensitivity of 77% and a specificity of 53%. Additionally, a score of 7 detects moderate dysphagia with a sensitivity of 81% and a specificity of 66%.
In addition to the 3-oz water test and EAT-10, Perry et al. [21] recently developed a clinical prediction model specific to ALS using commonly collected clinical variables to estimate a patient’s 3-month, 6-month, and 12-month risk of developing dysphagia. This model allows the healthcare team to input variables such as time since symptom onset and ALS functional rating scale total and subscale scores into an online calculator to generate a dysphagia-risk score. When using this model, the authors suggest that further swallowing evaluations may provide added value when the 3-month risk scores range from 15 to 50%.
Evaluations
In clinical practice, SLPs use a variety of evaluations to assess swallowing function in individuals with ALS [14•]. In a survey by Epps et al. [14•] from 2016 to 2017, tests used to diagnose dysphagia included clinical swallowing assessments, instrumental assessments, swallowing screens, and patient surveys. Clinical swallowing assessments, which likely included oral mechanism examinations and bolus trials, were the most commonly administered assessment among 49 SLPs throughout the USA. Additionally, Plowman et al.’s 2017 survey [22] listed routinely collected clinical outcomes such as weight and forced vital capacity. In both of these studies, the authors observed that the swallowing tests and outcomes collected varied among clinicians. Here, we have focused on assessments commonly used swallowing assessments in individuals with ALS.
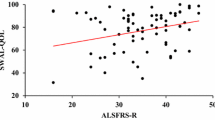
In a recent survey, the ALSFRS-R was the most consistently administered metric for tracking ALS disease progression [22]. The ALS-FRS is a 12-item patient-report scale about bulbar, fine motor, gross motor, and respiratory functions [47]. The bulbar subscale includes one question directly targeting swallowing function; lower scores indicate more significant impairment. Recently, a study by Chapin et al. [12], including 201 participants with ALS, found that an ALSFRS-R swallowing item score less than or equal to 3 was associated with penetration or aspiration with a sensitivity of 79% and a specificity of 60%.
The Center for Neurological Study Bulbar Function Scale (CNS-BFS) is a more recent patient-report scale rating the speech, swallowing, and salivation of individuals with ALS [25]. Higher scores are indicative of perceived worse impairment. The CNSBFS is well-correlated with the ALSFRS-R bulbar subscale, with a correlation coefficient of 0.90. Findings suggest that a score of 43 and above may indicate bulbar impairment. There are currently no validation studies comparing CNS-BFS to an instrumental swallowing assessment [30].
Unlike clinical assessments, instrumental assessments like VFSS and FEES allow SLPs to directly visualize the oropharyngeal structures involved in swallowing. As such, they may give clinicians more confidence in identifying swallowing impairments, selecting and probing appropriate compensatory strategies such as diet modifications, and determining the need for feeding tube placement [15, 30]. Additionally, they can serve as visual aids when educating patients and caregivers about aspiration risks. VFSS allows clinicians to observe physiological movements of the oropharyngeal structures from the front and lateral views using fluoroscopy. In contrast, FEES provides a superior view of the larynx and pharynx, including vocal fold appearance and movement, tissue integrity, and secretion management.
However, despite their benefits, research suggests that instrumental evaluations are not being utilized consistently for assessment in this population [15]. Reasons that healthcare professionals may not refer individuals with ALS for instrumental evaluation include a perceived lack of clinical utility due to the progressive nature of ALS and related dysphagia or new information not already obtained from clinical assessments [22]. Accessibility may be a barrier to VFSS as the facility may not be able to administer it; additionally, the patient may need an additional appointment to undergo the test. As for procedures, cleaning protocols required after FEES evaluations and scheduling limitations with VFSS may confine the number of assessments administered. Regardless of these barriers and the lack of formal guidelines regarding the timing of instrumental assessment, the literature suggests that SLPs should conduct instrumental evaluations early and repeatedly based on dysphagia indications [18, 27], with one study recommending VFSS at 6- and 12-month post-bulbar symptom onset. Frequent assessments allow SLPs and patients to comprehensively understand the progression of swallowing dysfunction.
To describe instrumental evaluation findings, clinicians may utilize standardized metrics. One specific to VFSS is the Modified Barium Swallow Impairment Profile (MBSImP), comprised of seventeen components categorized by the oral, pharyngeal, and esophageal phases [48]. Unfortunately, there appear to be no published studies that use MBSImP as recommended to characterize the physiologic impairments of swallowing function in ALS. However, Murono et al. [41] describe oral and pharyngeal phase impairments in 19 participants following an MBSImP-like framework. While they did not follow the administration protocol nor examine esophageal phase deficits, they found that those with bulbar symptoms of ALS had impaired bolus preparation/mastication and the initiation of the pharyngeal swallow during the oral phase. Additionally, participants with and without bulbar symptoms had impairments in bolus transport/lingual motion and oral residue. For the pharyngeal phase, those with bulbar symptoms had impairments in laryngeal elevation, anterior hyoid excursion, and tongue base retraction. Participants with and without bulbar symptoms had pharyngeal residue.
Murono et al. [41] also used the PAS to assess the incidence of penetration and aspiration observed on either VFSS or FEES in patients with ALS. The PAS is a clinician-rated scale that describes the location of penetration or aspiration, patient response, and airway clearance [49]. In their study, Murono et al. [41] found that among the 19 participants, those with bulbar symptoms had an average score of 1.50, and those without bulbar symptoms had an average score of 1.20. These participants were evaluated shortly after their ALS diagnosis, which may explain this low incidence of penetration and aspiration. Hence, the authors suggest that penetration and aspiration are uncommon in participants with and without bulbar symptoms at the initial diagnosis and that the onset of dysphagia occurs later in the disease process.
The Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) is another tool used to standardize instrumental assessment findings. It is a clinician-rated scale rating safety and efficiency that, when combined, result in a total score that indicates the severity of pharyngeal phase impairment ranging from 0 to 4. A higher total score indicates higher dysphagia severity [50]. Plowman et al. [6] used the DIGEST to describe dysphagia severity in 48 individuals with early-stage ALS undergoing active and sham expiratory muscle strength training trials. Their study defined total scores greater than 1, safety scores greater than or equal to 3, and efficiency scores greater than or equal to 1 as indicative of swallowing impairment.
While not a direct instrumental assessment of swallowing function, tongue movements during swallowing [19, 20] measured by electromagnetic articulography and tongue strength [10, 23] measured with the Iowa Oral Performance Instrument (IOPI) have been explored as potential biomarkers for swallowing impairment in this population. These studies suggest that changes in tongue movements during swallowing are present prior to the onset of swallowing impairment [19] and that tongue movements during swallowing had strong correlations with oral stage swallowing impairments as measured using MBSImP, PAS scale, and with patient-reported swallowing function [20]. Additionally, reduced tongue strength was found to be associated with inefficient and unsafe swallowing [10, 23].
Interventions
In their 2020 paper, Rogus-Pullia and Plowman [29••] call for a proactive approach to assessing and treating swallowing impairment in neurodegenerative disorders, including ALS, stating that current clinical practices are primarily reactive. Specifically, this approach requires the active collaboration of an interdisciplinary team, including SLPs at the time of ALS diagnosis, to help facilitate patient-specific care plans [29••]. In this section, we describe recent developments in interdisciplinary approaches to addressing dysphagia, some of which have promising implications as we move our practice forward.
Medical Interventions
Few pharmaceutical options exist for addressing ALS-related dysphagia [31]; one potential medication is dextromethorphan/quinidine (Nuedexta). Research by Smith et al. [7] found that compared to the placebo group, participants who received Nuedexta showed improvements in all subscales of the CNS-BFS, and half had at least a 1-point improvement on the ALSFRS-R bulbar subscale. However, the authors did not find significant differences in clinician ratings of speech and swallowing function between those who took Nuedexta and those who did not. This result suggests that Nuedexta may result in noticeable but minor patient-perceived improvements in speech, swallowing, and salivation; however, it is unclear if it can effectively improve swallowing.
Limited research has explored using laryngotracheal separation surgery, which reroutes the trachea to an anterior neck stoma, to prevent aspiration in the ALS population. Surgery may relieve discomfort and distress associated with aspiration for some individuals. Additionally, surgery may allow individuals to maintain oral intake for an average of 1 to 2 years and reduce the need for sputum suctioning [34]. However, although surgery prevents aspiration, it does not address the underlying mechanisms of swallowing impairment; thus, deficits in swallowing efficiency may persist [31, 34]. Additionally, due to the redirection of air from the vocal folds, individuals lose the ability to voice and communicate verbally through the glottis. Thus, as a result of surgery, patients who undergo this procedure become dependent on alternative and augmentative forms of communication.
Electrical Stimulation
In a recent survey [14•], 94% of SLPs reported that they would not use electrical stimulation (ES) for the ALS population. Indeed, ES may not be effective in neurodegenerative diseases like ALS due to underlying pathology [5]. For example, one study [5] examined the efficacy of pharyngeal ES in 20 participants with ALS and severe dysphagia. The participants were either part of the control group (standard speech therapy (ST)) or the intervention group (standard ST with pharyngeal ES). The control group focused on sensorimotor perception, postural changes, swallowing maneuvers, and modification of oral intake. The intervention group received treatment for 10 min across three consecutive days. While both groups improved in swallowing safety up to 3 weeks after the intervention, standard ST with pharyngeal ES was comparable to ST alone. In other words, pharyngeal ES did not add any additional benefits.
Exercise-based Interventions
Exercises focusing on muscle training and resistance for force generation [29••] have typically not been recommended for individuals with ALS since intensive exercise interventions theoretically might exhaust already weak muscles [31, 14•, 27]. For example, in Epps et al.’s survey [51], 85% of SLPs reported not using oromotor or laryngeal strengthening exercises as treatment in individuals with ALS. Additionally, a 2015 literature review by Plowman [51] found that instead of exercise, clinicians managing the ALS population focused primarily on safe swallowing strategies, diet modifications, feeding tubes, and compensatory strategies through adjusted postures. However, the 14 studies she analyzed indicated that early application of mildly-to-moderately intense limb and respiratory exercise might maintain function in individuals with ALS. Thus, Plowman suggests that mildly-to-moderately intense tasks may benefit swallowing.
Since respiratory and swallowing systems are closely related [26, 42], researchers have recently hypothesized that exercises aimed at respiratory musculature may simultaneously improve swallowing function [6, 51]. One such example is Expiratory Muscle Strength Training (EMST), an exercise routine centered around improving respiratory musculature strength. Participants in a single-case study [52] and a randomized controlled trial [6] demonstrated good tolerance of an 8-week home-based EMST program. In the randomized controlled trial, the authors found that the EMST group had maintained baseline global swallowing functioning and efficiency based on DIGEST scores compared to the sham group. The difference between the EMST and sham groups was significant for swallowing safety. Additionally, measures related to cough strength, like maximum expiratory pressure, improved in the EMST group. These studies suggest that EMST is safe and tolerable for individuals with early-to-middle stages of ALS and may be effective in improving swallowing function. However, it is unknown if EMST would be effective for those in the later stages or have severe dysphagia symptoms.
Artificial Nutrition Interventions
Due to weight loss and malnutrition in ALS [38, 39], maintaining adequate nutrition is vital. Therefore, when swallowing becomes impaired, individuals with ALS may choose to maintain nutrition orally, artificially through the placement of a feeding tube, or through a combination of both. Before resorting to more invasive means of nutrition, the patient and healthcare team may opt for an oral route [9] with SLP support and individualized counseling through standard care or with a dietitian [8]. However, often these strategies are only effective temporarily. Eventually, patients and their healthcare teams may need to consider feeding tubes as ALS progresses, especially when there is severe dysphagia and significant weight loss [11].
Regardless of the method, patient preference for nutritional management should take precedence [32] as we currently lack well-established guidelines for best practices in the nutritional management of this population. When making nutrition recommendations, the healthcare team should consider patient-related factors including psychological adjustment, need for control, understanding of ALS and related complications, and psychosocial aspects of eating [28]. Although artificial nutritional management discussions should begin early in the disease process to reduce weight loss [9], research suggests that individuals have varying positive and negative responses to early discussions on this topic [28]. To encourage patient engagement in the nutritional management plan, healthcare teams should empower the individual through education about ALS and related swallowing impairments. Zarotti et al. [28] recommend teaching strategies that reduce stress, misconceptions, and fears about eating. To illustrate these strategies, Seeber et al. [24] describe their experience counseling 28 participants, which included tailoring caregiver education topics based on observations or participant concerns like feeding tube use and management discussions.
A survey by Plowman et al. [22] suggests that rates of feeding tube use vary widely between healthcare providers and systems, with greater than 70% of patients receiving feeding tubes in some ALS multidisciplinary clinics and < 15% in others. This wide variation partly results from a lack of clarity on the benefits and risks of nutritional management strategies on outcomes and quality of life [4]. Much of the research has focused on the potential benefits of feeding tubes, including weight maintenance and survival. Some studies suggest that feeding tubes provide a survival benefit [4], while others suggest feeding tubes reduce life expectancy [16]. Similarly, evidence supporting weight maintenance for those with feeding tubes is weak, with only a few controlled studies showing a nutritional advantage of feeding tubes [4, 38, 53]. Research in this area is complex due to the heterogeneity of the disease process and the challenges of overcoming selection bias associated with dysphagia management decisions [29••].
Although the optimal timing for placement is unclear, the literature indicates that surgery should occur before the individual is in the advanced stages of ALS or has excessively reduced forced vital capacity [11]. The 2012 guidelines from the European Federation of the Neurological Sciences suggest that individuals with worsening respiratory status should consider percutaneous endoscopic gastrostomy tubes (PEG) even in the absence of dysphagia [54], as once dysphagia develops, it may be too late for placement. Benefits of early placement include potentially maximizing nutritional status and allowing for active patient participation in nutritional management interventions [33].
Conclusion
Shortcomings in care, uncertainty, and timing as well as practice variability affect the clinical assessment and management of dysphagia in individuals with ALS. Literature suggests that dysphagia assessment may be appropriate before patients even present with bulbar symptoms; however, most clinicians typically administer evaluations when symptoms manifest [29••]. Additionally, dysphagia evaluations vary widely among clinicians, and instrumental assessments are not used consistently, despite the high prevalence of swallowing dysfunction in this population. Although research using standardized instrumental evaluations of swallowing function is becoming increasingly more common in this population, it remains somewhat limited. As a result, our understanding of discrete changes in swallowing pathophysiology over the course of disease progression is limited [35]; thus, we cannot predict the progression of dysphagia. It is difficult to effectively and efficiently evaluate the impact of interventions aimed at slowing the onset or progression of dysphagia.
Likewise, many interventions directed at managing swallowing function in individuals with ALS remain primarily reactive rather than proactive [29•]. However, recent studies exploring the impact of EMST on swallowing function in individuals with early and middle stages of ALS [6] suggest that in the early stages of the disease process, (1) EMST may positively impact swallowing function, and (2) proactive, targeted exercise approaches may be promising.
In the absence of clinical best practices for dysphagia management in ALS, patient-centered care surrounding nutritional management strategies, including feeding tube placement and diet texture modifications, remains challenging, and practice patterns are highly variable. Further research in several areas of dysphagia assessment and intervention would advance our understanding and improve our management of swallowing impairment in ALS. To start, longitudinal studies using standardized instrumental assessment and measures would allow us to quantify swallowing changes over time as well as to describe and predict both meaningful and important changes in swallowing function. Determining meaningful and important change in swallowing function would also allow for us to more effectively and efficiently evaluate interventions aimed at prolonging swallowing function and begin to develop best practice guidelines for dysphagia management in this population. Additionally, further research centered around the development of proactive physiologically based exercise interventions to prolong swallowing function in the early stages of the disease process is greatly needed. Finally, in the absence of best practice clinical guidelines, research aimed at actively engaging patients in the dysphagia management decision-making process is critical to improving patient-centered care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Perry BJ, Nelson J, Wong JB, Kent DM. The cumulative incidence of dysphagia and dysphagia-free survival in persons diagnosed with amyotrophic lateral sclerosis. Muscle Nerve. 2021;64:83–6. https://doi.org/10.1002/mus.27244.
Higo R, Tayama N, Nito T. Longitudinal analysis of progression of dysphagia in amyotrophic lateral sclerosis. Auris Nasus Larynx. 2004;31:247–54. https://doi.org/10.1016/j.anl.2004.05.009.
Tabor L, Gaziano J, Watts S, Robison R, Plowman EK. Defining swallowing-related quality of life profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2016;31:376–82. https://doi.org/10.1007/s00455-015-9686-2.
Bond L, Ganguly P, Khamankar N, Mallet N, Bowen G, Green B, Mitchell CS. A Comprehensive Examination of Percutaneous Endoscopic Gastrostomy and Its Association with Amyotrophic Lateral Sclerosis Patient Outcomes. Brain Sci. 2019;9:223. https://doi.org/10.3390/brainsci9090223.
Herrmann C, Schradt F, Lindner-Pfleghar B, Schuster J, Ludolph AC, Dorst J. Pharyngeal electrical stimulation in amyotrophic lateral sclerosis: a pilot study. Ther Adv Neurol Disord. 2022;15:17562864211068394. https://doi.org/10.1177/17562864211068394.
Plowman EK, Tabor-Gray L, Rosado KM, Vasilopoulos T, Robison R, Chapin JL, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: results of a randomized, sham-controlled trial. Muscle Nerve. 2019;59:40–6. https://doi.org/10.1002/mus.26292.
Smith R, Pioro E, Myers K, Sirdofsky M, Goslin K, Meekins G, Yu H, Wymer J, Cudkowicz M, Macklin E, Schoenfield D, Pattee G, Macklin E. Enhanced bulbar function in amyotrophic lateral sclerosis: the Nuedexta treatment trial. Neurotherapeutics. 2017;14:762–72. https://doi.org/10.1007/s13311-016-0508-5.
Wills AM, Garry J, Hubbard J, Mezoian T, Breen CT, Ortiz-Miller C, et al. Nutritional counseling with or without mobile health technology: a randomized open-label standard-of-care-controlled trial in ALS. BMC Neurology. 2019;19:1–9. https://doi.org/10.1186/s12883-019-1330-6.
Essat M, Archer R, Williams I, Zarotti N, Coates E, Clowes M, et al. Interventions to promote oral nutritional behaviours in people living with neurodegenerative disorders of the motor system: a systematic review. Clin Nutr. 2020;39:2547–56. https://doi.org/10.1016/j.clnu.2019.11.015.
Borges ALF, Velasco LC, Ramos HVL, Imamura R, Roldão PMAC, Petrillo MVB, Costa CC. Association between dysphagia and tongue strength in patients with amyotrophic lateral sclerosis. Braz J Otorhinolaryngol. 2022;88:752–7. https://doi.org/10.1016/j.bjorl.2020.10.015.
Carbó Perseguer J, Madejón Seiz A, Romero Portales M, Martínez Hernández J, Mora Pardina JS, García-Samaniego J. Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis: mortality and complications. Neurología. 2019;34:582–8. https://doi.org/10.1016/j.nrleng.2018.01.002.
Chapin JL, Tabor Gray L, Vasilopoulos T, Anderson A, DiBiase L, Dallal York J, Robison R, Wymer J, Plowman EK. Diagnostic utility of the amyotrophic lateral sclerosis Functional Rating Scale-Revised to detect pharyngeal dysphagia in individuals with amyotrophic lateral sclerosis. PLoS One. 2020;15:e0236804. https://doi.org/10.1371/journal.pone0236804.
Donohue C, Tabor Gray L, Anderson A, DiBiase L, Chapin J, Wymer JP, Plowman EK. Discriminant ability of the Eating Assessment Tool-10 to detect swallowing safety and efficiency impairments. Laryngoscope. 2022;00:1–8. https://doi.org/10.1002/lary.30043.
Epps D, Kwan JY, Russell JW, Thomas T, Diaz-Abad M. Evaluation and management of dysphagia in amyotrophic lateral sclerosis: a survey of speech-language pathologists’ clinical practice. J Clin Neuromuscul Dis. 2020;21:135–43. https://doi.org/10.1097/CND.0000000000000281. This study surveyed SLPs throughout the United States from 2016 to 2017 regarding assessment and treatment trends for individuals with ALS from 2016 to 2017; it complements the survey by Plowman et al. published in 2017
(Focht) Garand KL, Suiter DM, Reyes S, Dallal York J, Chen IA. Aspiration screening in motor neuron disease: preliminary results from utilization of the Yale Swallow Protocol. Am J Speech-Lang Pathol. 2021;30:2693–9. https://doi.org/10.1044/2021_AJSLP-21-00092.
McDonnell E, Schoenfeld D, Paganoni S, Atassi N. Causal inference methods to study gastric tube use in amyotrophic lateral sclerosis. Neurology. 2017;89:1483 LP–1489. https://doi.org/10.1212/WNL.0000000000004534.
Onesti E, Schettino I, Gori MC, Frasca V, Ceccanti M, Cambieri C, et al. Dysphagia in amyotrophic lateral sclerosis: impact on patient behavior, diet adaptation, and riluzole management. Front Neurol. 2017;8:94. https://doi.org/10.3389/fneur.2017.00094.
Park YC, Lee JY, Lee JS, Park JS, Oh KW, Kim SH, Kim MJ. Characteristics of dysphagia based on the type of ALS in Korean patients evaluated using videofluoroscopic study: a retrospective analysis. Dysphagia. 2022;2022:1–9. https://doi.org/10.1007/s00455-022-10430-8.
Perry BJ, Martino R, Yunusova Y, Plowman EK, Green JR. Lingual and jaw kinematic abnormalities precede speech and swallowing impairments in ALS. Dysphagia. 2018;33:3840–7. https://doi.org/10.1007/s00455-018-9909-4.
Perry BJ, Stipancic KL, Martino R, Plowman EK, Green JR. Biomechanical biomarkers of tongue impairment during swallowing in persons diagnosed with amyotrophic lateral sclerosis. Dysphagia. 2021;36:147–56. https://doi.org/10.1007/s00455-020-10116-z.
Perry BJ, Nelson J, Wong JB, Kent DM. The Pooled Resource Open-Access ALS Clinical Trials Consortium. Predicting dysphagia onset in patients with ALS: the ALS dysphagia risk score. Amyotroph Lateral Scler Frontotemporal Degener. 2022;23:271–8. https://doi.org/10.1080/21678421.2021.1961805.
Plowman EK, Tabor LC, Wymer J, Pattee G. The evaluation of bulbar dysfunction in amyotrophic lateral sclerosis: survey of clinical practice patterns in the United States. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:351–7. https://doi.org/10.1080/21678421.2017.1313868.
Printza A, Boziki M, Triaridis S, Kiousi V, Arnaoutoglou M, Constantinidis J, Grigoriadis N. Tongue Strength, dysphagia questionnaire, pharyngeal secretions and FEES findings in dysphagia management in amyotrophic lateral sclerosis. Auris Nasus Larynx. 2021;48:672–82. https://doi.org/10.1016/j.anl.2020.10.007.
Seeber AA, Pols AJ, Hijdra A, Grupstra HF, Willems DL, de Visser M. Advance care planning in progressive neurological diseases: lessons from ALS. BMC Palliative Care. 2019;18:1–10. https://doi.org/10.1186/s12904-019-0433-6.
Smith RA, Macklin EA, Myers KJ, Pattee GL, Goslin KL, Meekins GD, et al. Assessment of bulbar function in amyotrophic lateral sclerosis: validation of a self-report scale (Center for Neurologic Study Bulbar Function Scale). Eur J Neurol. 2018;25:907–16. https://doi.org/10.1111/ene.13638.
Tabor-Gray L, Vasilopoulos T, Plowman EK. Differences in voluntary and reflexive cough strength in individuals with amyotrophic lateral sclerosis and healthy adults. Muscle Nerve. 2020;62:597–600. https://doi.org/10.1002/mus.27040.
Tye CB, Gardner PA, Dion GR, Simpson CB, Dominguez LM. Impact of fiberoptic evaluation of swallowing outcomes and dysphagia management in neurodegenerative disease. Laryngoscope. 2021;131:726–30. https://doi.org/10.1002/lary.28791.
Zarotti N, Coates E, McGeachan A, Williams I, Beever D, Hackney G, et al. Health care professionals’ views on psychological factors affecting nutritional behaviour in people with motor neuron disease: a thematic analysis. Brit J Health Psych. 2019;24:953–69. https://doi.org/10.1111/bjhp.12388.
Rogus-Pulia NM, Plowman EK. Shifting tides toward a proactive patient-centered approach in dysphagia management of neurodegenerative disease. Am J Speech Lang Pathol. 2020;29:1094–109. https://doi.org/10.1044/2020_AJSLP-19-00136. This perspective article advocates the need for proactive assessment and treatment of dysphagia in motor neuron disease, providing its importance and suggesting ways to move the field in the active direction as supported by current research.
Yunusova Y, Plowman EK, Green JR, Barnett C, Bede P. Clinical measures of bulbar dysfunction in ALS. Front Neurol. 2019;10:1–11. https://doi.org/10.3389/fneur.2019.00106.
Britton D, Karam C, Schindler JS. Swallowing and secretion management in neuromuscular disease. Clin Chest Med. 2018;39:449–57. https://doi.org/10.1016/j.ccm.2018.01.007.
Everett EA, Pedowitz E, Maiser S, Cohen J, Besbris J, Mehta AK, Chi L, Jones CA. Top ten tips palliative care clinicians should know about amyotrophic lateral sclerosis. J Palliat Med. 2020;23:842–7. https://doi.org/10.1089/jpm.2020.0046.
Marques V, Orsini M, Fiorelli R, Bastos VH, Teixeira S, Fiorelli S, et al. Early gastrostomy associated with speech therapy in patients with amyotrophic lateral sclerosis. Fisioterapia Brasil. 2018;19:414–6. https://doi.org/10.33233/fb.v19i3.2437.
Soga T, Suzuki N, Kato K, Kawamoto-Hirano A, Kawauchi Y, Izumi R, et al. Long-term outcomes after surgery to prevent aspiration for patients with amyotrophic lateral sclerosis. BMC Neurol. 2022;22:1–8. https://doi.org/10.1186/s12883-022-02619-z.
Waito AA, Valenzano TJ, Peladeau-Pigeon M, Steele CM. Trends in research literature describing dysphagia in motor neuron diseases (MND): a scoping review. Dysphagia. 2017;32:734–47. https://doi.org/10.1007/s00455-017-9819-x.
Dang D, Dearholt S, Bissett K, Ascenzi J, Whalen M. Johns Hopkins evidence based practice for nurses and healthcare professionals: model and guidelines. 4th ed. Indiana: Sigma Theta Tau International; 2022.
Chen A, Garrett CG. Otolaryngologic presentations of amyotrophic lateral sclerosis. Otolaryng Head Neck. 2005;132:500–4. https://doi.org/10.1016/j.otohns.2004.09.092.
Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53:1059–63. https://doi.org/10.1212/WNL.53.5.1059.
Limousin N, Blasco H, Corcia P, Gordon PH, De Toffol B, Andres C, Praline J. Malnutrition at the time of diagnosis is associated with a shorter disease duration in ALS. J Neurol Sci. 2010;297:36–9. https://doi.org/10.1016/j.jns.2010.06.028.
Qutub K, Lacomis D, Albert SM, Feingold E. Life factors affecting depression and burden in amyotrophic lateral sclerosis caregivers. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:292–7. https://doi.org/10.3109/21678421.2014.886699.
Murono S, Hamaguchi T, Yoshida H, Nakanishi Y, Tsuji A, Endo K, et al. Evaluation of dysphagia at the initial diagnosis of amyotrophic lateral sclerosis. Auris Nasus Larynx. 2015;42:213–7. https://doi.org/10.1016/j.anl.2014.10.012.
Plowman EK, Watts SA, Robison R, Tabor L, Dion C, Gaziano J, et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia. 2016;31:383–90. https://doi.org/10.1007/s00455-015-9687-1.
Plowman EK, Tabor LC, Robison R, Gaziano J, Dion C, Watts SA, Gooch C. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2016;28:85–90. https://doi.org/10.1111/nmo.12700.
Donohue C, Tabor Gray L, Chapin J, Anderson A, DiBiase L, Wymer JP, Plowman EK. Discriminant ability of the 3-ounce water swallow test to detect aspiration in amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2021;34:e14310. https://doi.org/10.1111/nmo.14310.
Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emergency. 2016;4:111–3. https://doi.org/10.22037/aaem.v4i2.232
Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, Leonard RJ. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117:919–24. https://doi.org/10.1177/000348940811701210.
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. https://doi.org/10.1016/s0022-510x(99)00210-5.
Martin-Harris B, Humphries K, Garand KL. The Modified Barium Swallow Impairment Profile (MBSImPTM©) – innovation, dissemination and implementation. Perspectives. 2017;2(13):129–38. https://doi.org/10.1044/persp2.SIG13.129.
Rosenbek J, Robbins J, Roecker E, Coyle J, Wood J. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. https://doi.org/10.1007/BF00417897.
Hutcheson KA, Barrow MP, Barringer DA, Knott JK, Lin HY, Weber RS, et al. Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): scale development and validation. Cancer. 2016;123:62–70. https://doi.org/10.1002/cncr.30283.
Plowman EK. Is there a role for exercise in the management of bulbar dysfunction in amyotrophic lateral sclerosis? J Speech Lang Hear Research. 2015;58:1151–66. https://doi.org/10.1044/2015_JSLHR-S-14-0270.
Tabor LC, Rosado KM, Robison R, Hegland K, Humbert IA, Plowman EK. Respiratory training in an individual with amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2016;3:819–23. https://doi.org/10.1002/acn3.342.
Mazzini L, Corrà T, Zaccala M, Mora G, Del Piano M, Galante M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. J Neurol. 1995;242:695–8. https://doi.org/10.1007/BF00866922.
Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) – revised report of an EFNS task force. Eur J Neurol. 2012;19:360–75. https://doi.org/10.1111/j.1468-1331.2011.03501.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kao, T.H., Perry, B.J. The Current State and Future Directions of Swallowing Care in Amyotrophic Lateral Sclerosis. Curr Phys Med Rehabil Rep 11, 199–211 (2023). https://doi.org/10.1007/s40141-023-00396-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-023-00396-5