Abstract
Purpose of Review
To describe the cardiac changes related to COVID-19 in athletes, in addition to presenting the current recommendations for cardiac assessment and return to sport after COVID-19 infection.
Recent Findings
The current state of the art suggests that myocarditis and pericarditis are the main cardiac pathologies related to the COVID-19 infection in athletes even after recovery. The criteria for determining and evaluating cardiac conditions are still discussed, as well as what stage of infection do cardiomyopathies occur. Return to sport should be aligned with cardiovascular risk stratification.
Summary
Cardiac changes related to COVID-19 infection have drawn the attention in the sports medicine field, while some questions about the course of the disease and its relationship with physical performance in athletes are still under investigation. In addition, feasible assessment techniques for cardiac assessments should be explored in the future.
Similar content being viewed by others
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2 or COVID-19) that emerged in December 2019 quickly spread across the world, officially becoming a global pandemic in March 2020. The COVID-19 differs from other respiratory virus due to high contamination in addition to rapid spread capacity, with several implications in the sports world regarding the spread of SARS-CoV-2 among athletes and sports teams. It is known that COVID-19 affects mainly the respiratory tract and has a clinical characteristic from a mild flulike illness to potentially lethal acute respiratory syndrome or pneumonia [1]. However, it is also associated with several cardiovascular complications, specific arrhythmias, myocardial injury, and other cardiovascular disease, with potentially fatal cardiovascular events in athletes and non-athletes [2].
Previous data suggest that athletes are susceptible to cardiovascular complications after COVID-19 infection, including myocarditis, pre-myocardial injury, increased arterial stiffness, and decreased vascular function [2, 3], raising concern about myocardial inflammation as an additional cause of cardiac damage from COVID-19 in this population [4, 5]. In order to face the COVID-19 spread and prevent the collapse of the health system, some rigorous strategies based on social distancing and adherence to home quarantine have been practiced worldwide. Strategies to contain the spread of the virus have included cancellation of sporting events and seasons from amateur to professional levels. Impacting the physical activity levels, cardiorespiratory fitness, and physical performance in athletes [2].
Nowadays, relaxation of lockdown orders in most countries enabled athletes to return to training and competition routines. However, several recommendations have been proposed in order to minimize the increased risk for malignant arrhythmias among athletes exposed to COVID-19, during this return to sport phase [6••]. There are still several clinical challenges in dealing with COVID-19–related cardiac changes in athletes, such as who to screen; how to screen; how to interpret the clinical tests; the prevalence of COVID-19 cardiac involvement; the screen test sensitivity and specificity in order to detect myocardial pathology; and return to sport and training safety [2, 6••]. Therefore, this study aims to describe and revise the main cardiac alterations related to COVID-19 in elite and recreational athletes, as well as current recommendations for clinical screening, and safe return to sports routine.
Cardiac Changes
Although there is no widely accepted definition about the clinical relevance of myocardial injury secondary to COVID-19 infection among athletes in competitive sports, several studies have emerged in an attempt to elucidate the relationship of cardiac changes with COVID-19 in these individuals [6••]. A recent systematic review that investigated cardiovascular complications in athletes with COVID-19, suggested that a variety of cardiovascular complications were reported in athletes, such as myocarditis, pericardial effusion, effuse viral pericarditis, and myocardial edema [4]. Moreover, these abnormalities have been found to be persistent post COVID-19 recovery according to cardiac magnetic resonance (CRM) results. The most frequent cardiac change was myocarditis followed by regional scar and pericardial enhancement [4]. While the myocardial inflammation or fibrosis after COVID-19 recovery is also observed in competitive athletes, which is the major cause of sport-related sudden cardiac death and can happen regardless of a normal ventricular output [7].
Myocarditis, defined as extensive inflammation of the myocardium, is a mixture of myocardial damage and systemic immune-inflammatory response, may involve the pericardium and characterized by autoptic finding of lymphocytic infiltrates. The myocardiocytes (responsible for the electrical conduction) is also affected by the inflammation, which can lead to conduction disturbances, blocks and malignant ventricular arrhythmias, which in turn can lead to cardiac arrest [8]. In COVID-19 patients, the mechanism of myocardial injury has several etiologies, such as direct viral infection via binding of the angiotensin-converting enzyme 2 (ACE2) receptors on myocardial and endothelial surfaces, virus-induced immune reaction that releases too many cytokines into the blood quickly (cytokine storm) leading to ischemia and necrosis. Vessel inflammation and a hypercoagulable state can also contribute to ischemia and thrombotic complications [2, 9]. Increase on cardiac biomarkers, such as troponin-I and B-type natriuretic peptide (BNP), are common in more severe cases, as are markers of systemic inflammation, including C-reactive protein (CRP) [10].
A recent study explored the cardiac involvement in college student athletes who recovered from uncomplicated COVID-19 [11]. The authors found that one-half of the patients showed cardiac examination abnormalities consistent with pericardial late enhancement with pockets of pericardial effusion and patchy or a diffuse pattern of myocardial segmental strain abnormalities, suggesting that this may be related to a subacute or convalescing phase of pericarditis. Curiously, endothelial injury associated with intracellular COVID-19 virus has been associated to new vessel growth, a mechanism referred to as intussusceptive angiogenesis. Possibly due to susceptibility of pericardial mesothelium cells to COVID-19 virus secondary to angiotensin-converting enzyme receptor activity coupled with inflammation and angiogenesis may explain the high prevalence of pericardial late enhancements [12, 13]. Although the clinical relevance of these findings is not yet fully elucidated, these results suggest that mild or asymptomatic COVID-19 is not a benign condition, taking into account that more than half of young individuals showed subclinical myocardial and pericardial diseases, even after recovery from COVID-19.
Although an enhanced cardiorespiratory fitness in athletes seems to be protective against a severe COVID-19 infection, since usually athletes have showed none or mild symptoms during infection and after recovery, the cardiovascular system can still be silently affected [14, 15]. Comparison of vascular changes in male elite athletes recovering from COVID-19 versus uninfected individuals showed a decline of vascular and endothelial function in athletes despite the excellent fitness levels of the participants and the mild course of COVID-19 infection. These results suggest that the infection may affect not only cardiac function but may detrimentally affect aortic reservoir function, resulting in an increase in arterial stiffness, consequently, increasing cardiac work [3].
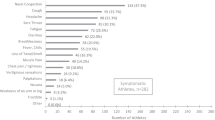
Clinical Features and Screening
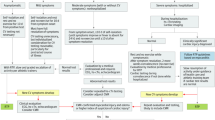
Early recognition and continuous monitoring of cardiac function are essential to prevent cardiac complications. General short-term symptoms may include sore throat, myalgia, dyspnea, fever, while some of the athletes are asymptomatic [4, 5]. Meanwhile, the long-term COVID-19 symptoms (post-recovery) may be nonspecific and include cough, tachycardia, severe fatigue, ventricular arrhythmias, chest pain, decreased exercise tolerance and depression [16]. In terms of cardiovascular assessment, it is important to include specialized blood panel, resting electrocardiogram (ECG), 24-h ECG, echocardiogram, cardiopulmonary exercise test, assessment of flow-mediated dilation and CMR [3, 4]. The CMR has been used to detect congestive heart failure, cardiac tamponade, and acute myocardial infarction. Meanwhile, the elevation of troponin is an indicator of silent myocardial inflammation. Therefore, all the clinical assessments must form part of a monitoring plan before and during the sport return. In addition, it is important for health professionals to understand that there are several physiological cardiac changes expected as adaptation to physical exercise, and cardiac changes related to COVID-19 infection, which in turn may overlap those sport-related changes as shown in Fig. 1 [2, 17].
Some authors classified myocardial injury as either [1] CMR T1 changes or late gadolinium enhancement (LGE) + T2 changes; or [2] CMR T2 changes + at least one supportive finding (EF reduced, pericardial effusion, pericardial enhancement, or troponin > 99% upper limit of normal) [18]. Moreover, CMR T1 changes or LGE + at least one supportive finding is classified as probable myocardial injury. The diagnosis of acute myocarditis should also include a clinical syndrome of acute heart failure, angina-type chest pain, or known myopericarditis of less than 3-month duration. In addition to unexplained elevation of troponin, ECG changes, arrhythmia or high-grade atrioventricular block, systolic dysfunction or regional wall motion abnormalities, or pericardial effusion [19••].
In addition to the aforementioned tests, the exercise test is also a valuable tool in the assessment of post-COVID-19 athlete’s cardiac responses. Exercise testing is an important diagnostic and prognostic exam in the management of the athletes after COVID-19. However, exercise testing is contraindicated during the acute phase, not only to avoid an exacerbated immune response, but also to prevent contamination of the laboratory staff. Exercise test should be considered if the athlete has new or persistent symptoms during physical practice, once the athlete is no longer infectious and active myocarditis is ruled out [20]. Cardiopulmonary exercise test (CEPT) should be performed in those individuals with undifferentiated dyspnea or exertional intolerance, since CEPT can identify both cardiac and pulmonary sequelae of COVID-19. According to the current guidelines, exercise test should also be assessed in those athletes with COVID-19–associated myocarditis after 3 to 6 months, as part of return to play risk stratification [19••].
Safe Return to Play
In the acute COVD-19 infection, physical exercise practice may promote an increase in the spread and replication of the virus and enhance the immune-inflammatory response, consequently, leading to an increased risk of myocardial damage and myocardiocyte necrosis. According to the necrosis extension, the scar tissue that will occur because of myocarditis, can potentially rise the risk of atrial and/or ventricular tachyarrhythmias [21]. Therefore, practicing sports during this period is not recommended.
Recent guidelines proposed in October 2020 by experts, recommend screening athletes for cardiac involvement due to COVID-19 infection, especially for a safe return to sport [6••]. The consensus proposes an algorithm for sport return based on the severity of the infection and the presence of cardiac symptoms [6••]. Those athletes with asymptomatic or mild COVID-19 infection without cardiovascular symptoms may return to play gradually after self-isolation period. Moreover, non-hospitalized individuals with moderate symptoms should undergo ECG, troponin, and echocardiogram exams. Finally, athletes with severe infection hospitalized, troponin and echocardiogram tests should be performed during the hospitalization period and CMR should be considered. Moreover, in case of irregular test results or new cardiovascular symptoms, further investigation should be performed, as well as CMR when feasible [6••]. In case of normal CMR results but clinical suspicion of cardiac involvement remains, serial test may be considered [22]. A summary of the return to practice algorithm for adult athletes in competitive sports is shown in Fig. 2 [6••].
Coronavirus Disease 2019 (COVID-19) Return-to-Play Algorithm for Adult Athletes in Competitive Sports proposed by [6••]. CV: cardiovascular; hs-cTn: high-sensitive troponin-I; RTP: return to play; Mild symptoms: anosmia, ageusia, headache, mild fatigue, mild upper respiratory tract illness, and mild gastrointestinal illness; Moderate: persistent fever, chills, myalgias, lethargy, dyspnea, and chest tightness; CV symptoms: dyspnea, exercise intolerance, chest tightness, dizziness, syncope, and palpitation
It is important to note that the scheme shown in Fig. 2 is based on an algorithm for returning to sport for adult athletes. The guidelines to return to play include a separate scheme for master athletes, which deserve more attention, since COVID-19 cardiac involvement is probably higher in these athletes, especially when cardiovascular risk factors are present [6••].
According to the literature, the number of athletes who are barred from returning to sport is low. However, there are still some literature gaps, especially regarding the clinical significance of cardiac assessments. For example, the clinical significance of isolated pericardial LGE or transient isolated myocardial edema with no symptoms, negative biomarkers, normal ECG, and echocardiogram. In addition, eventually myocardial inflammation detection may be missed based on the current strategy, since some athletes may develop delayed clinical manifestations. Another possible limitation of the proposed scheme is the feasibility of the CMR exam; despite its great importance, the exam requires expensive equipment, and an experienced evaluator for proper interpretation [2, 6••].
Perspectives
Although cardiac changes due to COVID-19 infection are well reported in the literature. The real impact of COVID-19–related cardiac changes on athletic performance in the short, medium, and long term is not yet fully established. The methods of cardiac screening and when it should be done are still discussed, as well as the feasibility of the methods. Future studies should investigate the criteria for scanning cardiac changes, as well as new approaches such as cardiac autonomic assessment. Some studies suggest that cardiac autonomic impairment is also involved during COVID-19 infection and is related to worse prognosis [23]. The study of cardiac autonomic modulation can be easily performed through non-invasive assessment based on heart rate variability, which is a predictor index of morbidity and mortality in patients with cardiovascular disease. A recent study suggested that the cardiac autonomic nervous system imbalance, assessed by heart rate variability, may be a prominent feature of acute COVID-19 [23]. However, to the best of my knowledge, there are no studies on this relationship in athletes infected with COVID-19.
In addition, the decision on returning to sport must be based on a shared-decision approach. In other words, it should take into account not only medical team opinion, when there is absence of serious quantified risk. That is, isolated abnormal findings (such as mildly elevated levels of hd-cTn or nonspecific imaging findings), in these cases should require shared decision-making with the athlete in order to reach a balance between the clinician’s risk estimation and the patient’s tolerance for risk assumption to then establish sports eligibility [6••].
Conclusion
The COVID-19 pandemic has affected sports practice around the world, affecting the routine of elite and recreational athletes. The symptoms of the disease are variable throughout the different stages of the disease and cardiac involvement is something present in these individuals. The cardiac changes related to COVID-19 infection have drawn the attention of coaches and health professionals in the cardiology field, requiring special attention for these athletes, since these changes may be associated with cardiovascular events, especially in the return to sport phase. The knowledge of COVID-19–related cardiac changes can guide cardiology professionals in cardiovascular risk stratification. Regardless of the stage of infection, risk stratification for CV should be implemented in the routine of athletes from the hospital phase to the late phase. Moreover, the athlete’s team must be trained to act in cardiac emergency situations that may eventually occur during sports practice.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Goergen J, Bavishi A, Eimer M, Zielinski AR. COVID-19: the risk to athletes. Curr Treat Options Cardiovasc Med. 2021;23(11):68.
Bauer P, Kraushaar L, Dörr O, Keranov S, Nef H, Hamm CW, et al. Vascular alterations among male elite athletes recovering from SARS-CoV-2 infection. Sci Rep. 2022;12(1):8655.
Alosaimi B, AlFayyad I, Alshuaibi S, Almutairi G, Alshaebi N, Alayyaf A, et al. Cardiovascular complications and outcomes among athletes with COVID-19 disease: a systematic review. BMC Sports Sci Med Rehabil. 2022;14:74.
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–8.
•• Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, et al. Coronavirus disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219–27. Guidelines for sport return.
Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 Myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143(6):609–12.
Topol EJ. COVID-19 can affect the heart. Science. 23 de outubro de. 2020;370(6515):408–9.
Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173(12):1025–7.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93.
Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. JACC Cardiovasc Imaging. 2021;14(3):541–55.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM. Son MBF et al Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334–46.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8.
Brawner CA, Ehrman JK, Bole S, Kerrigan DJ, Parikh SS, Lewis BK, et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96(1):32–9.
Zbinden-Foncea H, Francaux M, Deldicque L, Hawley JA. Does high cardiorespiratory fitness confer some protection against proinflammatory responses after infection by SARS-CoV-2? Obes Silver Spring Md. 2020;28(8):1378–81.
Wilson MG, Hull JH, Rogers J, Pollock N, Dodd M, Haines J, et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br J Sports Med. 2020;54(19):1157–61.
Brosnan MJ, Rakhit D. Differentiating athlete’s heart from cardiomyopathies - the left side. Heart Lung Circ. 2018;27(9):1052–62.
Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–66.
•• Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NAM, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273-280. Guidelines for the management of athletes with cardiovascular abnormalities.
Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. JACC Cardiovasc Imaging. 2020;13(12):2635–52.
Tasca JS, Bianchi G, Girardello A, Lucchini A, Cappelli C. Cardiac involvement in athletes infected by SARS COV-2 disease. Sci Sports. 2022;37(3):167–75.
Eichhorn C, Bière L, Schnell F, Schmied C, Wilhelm M, Kwong RY, et al. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):494–507.
Scala I, Rizzo PA, Bellavia S, Brunetti V, Colò F, Broccolini A, et al. Autonomic dysfunction during acute SARS-CoV-2 infection: a systematic review. J Clin Med. 2022;11(13):3883.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Cardiovascular Emergencies in Sport: Epidemiology, Prevention and Treatment
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Abreu, R.M. Cardiac Changes Related to COVID-19 in Athletes: A Brief Review. Curr Emerg Hosp Med Rep 10, 143–148 (2022). https://doi.org/10.1007/s40138-022-00252-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-022-00252-1