Abstract
Purpose of Review
The aim was to synthesize key findings regarding the use of functional MRI (fMRI) to assess olfactory dysfunction (OD), and thus, to evaluate whether fMRI could be a reliable clinical diagnostic tool.
Recent Findings
In response to olfactory stimulation, patients with quantitative OD display reduced activation in olfactory-related brain regions but also stronger activation in non-olfactory brain areas. Parosmic patients also seem to show both weaker and higher brain signals. As to trigeminal chemosensory system, fMRI suggests that central processing may be declined in patients with OD. Functional connectivity studies report a possible correlation between altered neuronal connections within brain networks and olfactory performances.
Summary
fMRI emerges as a valuable and promising objective method in OD evaluation. Yet, its high inter-individual variability still precludes its routine clinical use for diagnostic purpose. Future research should focus on optimizing stimulation paradigms and analysis methods.
Similar content being viewed by others
Introduction
It is widely acknowledged that olfactory disorders require a thorough clinical assessment. The most commonly used method to assess olfactory function is psychophysical evaluation. Indeed, these tests offer the advantages of being validated for clinical use, easy to administer, and there are normative values to which it is possible to refer. However, since psychophysical tests rely on patients’ answers and collaboration, they are subject to response bias, and therefore, their reliability may be affected by several factors such as patients’ culture, their goodwill, or even malingering. This is particularly problematic in medico-legal evaluation.
On the other hand, electrophysiological tests, such as olfactory event-related potentials, allow for an objective evaluation of the olfactory function and only demand a small collaboration from the subjects. Olfactory event-related potentials offer the advantage of a good time resolution, but their contrast spatial resolution is relatively weak. Moreover, electrophysiological tests require both expensive and specific equipment (i.e., an olfactometer which allows highly-controlled stimuli with rapid rise-times) and a high expertise, making them restricted to highly specialized centers.
The classical work-up of patients with olfactory disorders also relies on neuroimaging of the olfactory system. The main aims of neuroimaging are to identify the cause of olfactory dysfunction (OD) and to exclude intracranial tumors. Structural MRI comes as the gold-standard imaging modality to assess the olfactory system. Notably, this technique may give valuable information in case of congenital anosmia or post-traumatic OD since it will show some typical patterns that allow to confirm the suspected etiology (i.e., aplastic or hypoplastic olfactory bulbs and olfactory sulcus in congenital anosmia, specific post-traumatic lesions). However, while structural MRI provides interesting information about the morphology of the olfactory system, it does not offer any information about its functionality.
The neuroimaging technique of choice for testing the functional integrity of the olfactory system is functional MRI (fMRI) which is based on the measurement of changes in blood flow. The advantages of fMRI are its good spatial resolution and non-invasive character. This method offers the possibility to study the processing of olfactory information and constitutes an objective assessment of olfactory functioning. Although fMRI has significantly increased our knowledge of human olfactory processing, its clinical value on an individual level remains somewhat debatable [1••].
In this review, we will summarize brain imaging findings in patients with olfactory disorders and discuss their usefulness in a clinical environment.
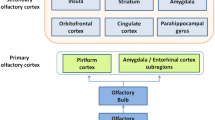
Central Olfactory Processing
Olfactory processing starts peripherally at the level of the olfactory epithelium, where odorants bind to olfactory sensory neurons (OSNs). OSNs project their axons onto the olfactory bulb (OB) where they synapse with second-order olfactory neurons, known as mitral/tufted cells. These second-order neurons project to diverse brain areas, such as the piriform cortex, the entorhinal cortex, and the amygdala. These areas are collectively referred to as the primary olfactory cortex [1••, 2]. Neurons from these primary olfactory cortex regions project to various other brain regions such as the orbitofrontal cortex, hippocampus, parahippocampal gyrus, insula, cingulate cortex, thalamus, and amygdala. These latter areas are commonly referred to as the secondary olfactory cortex [1••, 2]. A multitude of fMRI studies performed in healthy normosmic people found that olfactory stimuli activate these primary and secondary olfactory cortices areas. Importantly, however, the pattern of activation is modulated by diverse experimental factors like task instructions and/or stimulus qualities [3,4,5,6,7].
Functional MRI
fMRI uses blood-oxygen-level dependent (BOLD) contrast as an indicator of cerebral activity. Changes in the BOLD response can be investigated either after olfactory stimulation or at rest. The latter condition, called resting-state fMRI (rsfMRI), evaluates spontaneous fluctuations in the BOLD signal in the absence of an olfactory stimulus, looking in particular at regional interactions and brain networks in the absence of an externally imposed task or stimulus.
It is important to mention that the majority of studies evaluated heterogeneous groups of patients, in the sense that they involved patients with diverse etiologies of OD (i.e., acquired OD such as post-infectious or post-traumatic OD, congenital anosmia). Only few studies focused on patients with a specific disease. In the following, we will explicitly mention when a study refers to a specific group of patients, while the generic term “OD” will be used to refer to a heterogeneous group of patients.
Olfactory-Related Brain Activation in Patients with OD
Studies performed in OD patients have shown that olfactory stimuli activate the same brain regions as observed in normosmic controls, such as the piriform cortex, amygdala, thalamus, insula, putamen, caudate region, cingulate, and orbitofrontal cortices. However, these activations are significantly weaker in OD patients [1••, 8,9,10,11, 12•], possibly reflecting decreased olfactory perception [9]. Interestingly, a study with post-traumatic OD patients demonstrated that, not only patients had significantly decreased brain activation in comparison to controls, but also that these responses were related to the hedonic quality of the stimulus [11]. Indeed, unpleasant olfactory stimulation (B-mercaptoethanol) led to reduced activation of the primary and secondary olfactory cortices and the limbic system compared to control subjects. Whereas, after pleasant stimulation (citral), reduced activation was only observed in the left frontal subgyral region compared to controls. In the same vein, Pellegrino et al. found that hyposmic patients do not display amygdalar activation in response to odorant stimulation [9]. Since the amygdala is involved in the encoding of odor pleasantness and intensity [13], the absence of amygdalar activation in hyposmic patients might be explained by reduced odor intensity perception, leading to reduced pleasantness in OD patients.
Patients with OD not only have weaker activations in olfactory-related brain regions; they also present stronger activation in non-olfactory brain regions such as the posterior cingulate cortex [9, 12•]. Since the posterior cingulate cortex is involved in memory-odor associations, odor memory retrieval [14], and semantic memory processes [15, 16], a stronger activation in this area in hyposmic patients could indicate more demanding olfactory memory processing. Another study in post-traumatic OD patients also found higher activation at the level of the mediodorsal thalamus, a brain area responsible for attention to odor stimuli, and of the ventromedial prefrontal cortex [12•].
Besides “quantitative” OD such as anosmia or hyposmia, patients may also complain of “qualitative” OD such as parosmia or phantosmia. Parosmias are usually associated to a quantitative OD and are mainly found in patients with post-infectious OD [17]; they are more frequently reported in patients with moderate olfactory dysfunction or during recovery [18]. A majority of patients report that they have a negative hedonic valence in response to odorants [18]. Consequently, parosmias usually have a more severe impact on patients’ quality of life and lead to a more severe impairment in everyday life. Although the exact cause of parosmia remains unclear, it is generally believed that both peripheral and central mechanisms play a role in its ontogenesis. To study the role of the central nervous system in parosmias, Iannilli et al. compared fMRI activation patterns after olfactory stimulation in hyposmic patients with and without parosmia [19]. Although both groups were similar in terms of quantitative olfactory function, parosmic patients had reduced activation of several olfactory-related areas, including the right medial orbitofrontal cortex, left anterior cingulate cortex, left parahippocampal gyrus, and right insula. In contrast, parosmic patients had stronger activations in the left thalamus and right putamen. Previous studies have shown that the thalamus is associated with directed attention in relation to the olfactory stimuli, while the putamen has been implicated in the perception of disgust [19,20,21].
Functional MRI studies also evaluated trigeminal chemosensory processing in OD patients. It is well known that olfactory and trigeminal chemosensory systems closely interact with each other to give a global chemosensory perception. In normosmic controls, fMRI studies indicated that trigeminal and olfactory stimulation leads to the activation of overlapping areas, including the piriform cortex, the orbitofrontal cortex, and the insula [22]. Hence, trigeminal stimulation produces activation in areas typically involved in olfactory processing. However, some areas are preferentially activated by trigeminal stimulation, such as the primary and secondary somatosensory cortices, mid-cingulate and anterior orbitofrontal cortices, dorsomedial thalamus, cerebellum, and brain stem. In contrast, pure olfactory stimuli show superior activation of the amygdala and medial orbitofrontal cortex [22]. Psychophysical studies have shown that OD is associated with modifications of trigeminal chemosensory sensitivity. Indeed, patients with acquired OD have lower performance in trigeminal detection thresholds or lateralization tasks in comparison to controls [23,24,25]. Trigeminal chemosensory ERPs are also reduced in patients with acquired OD [26]. Congruently, fMRI studies in response to trigeminal stimulation revealed reduced activation of the prefrontal cortex, the primary somatosensory cortex, and the insular cortex in OD patients, in comparison to controls. This result suggests that the central processing of trigeminal stimuli is impaired in OD patients [27].
In post-traumatic OD patients, a correlation was found between poor psychophysical olfactory performance and decreased BOLD activation in the left insula [11], frontal operculum, and anterior insula [12•].
Functional Connectivity
Functional MRI also allows to measure the functional connectivity (FC) between brain areas and networks. FC provides a measurement of the temporal correlation of the neuronal activity between different brain regions, hence, reflecting the functional communication between them [28]. FC can be measured either during tasks or at a resting state [29].
Task-Induced FC Studies
FC analyses during olfactory and/or trigeminal chemosensory stimulation revealed three major networks that overlapped significantly: (1) an olfactory network (putamen/caudate nucleus, piriform cortex, entorhinal cortex, amygdala, thalamus), (2) a somatosensory network (primary and secondary somatosensory cortices, insula), and (3) an integrative network (orbitofrontal cortex, insula, inferior parietal lobule, middle and superior temporal gyrus) [30]. Although normosmic and anosmic patients use the same network to process chemosensory (olfactory and/or trigeminal) information, they exhibit altered FC. Indeed, patients had fewer connections in comparison to controls, mainly in the olfactory network [30]. Interestingly, in the same study, the authors compared the three networks before and after olfactory training in the anosmic group. Olfactory training consists of a regular and repeated daily exposure to odorants; it is currently considered the gold-standard treatment of OD. Studies agree that olfactory training improves olfactory function [31], but little is known about its underlying mechanisms. After 12 weeks of olfactory training, patients had a significant improvement in olfactory sensitivity and had increased signal intensity in the three networks, especially in the olfactory and somatosensory networks. Moreover, there was also increased FC in all networks, particularly in the olfactory network, suggesting a recovery of olfactory-specific functional connections after olfactory training [30].
Another study investigated how the decline in olfactory function affects neural activation patterns and networks. For that purpose, the authors evaluated patients with OD (anosmia and hyposmia) and investigated the correlation between olfactory function and neural activation and networks [32]. It was shown that olfactory stimulation activates the piriform cortex, but this activation was not correlated with psychophysical olfactory performance. Regarding the networks, olfactory stimulation recruited a sensory processing network comprising the insula, thalamus, piriform cortex, cingulate gyrus, and putamen. Interestingly, the recruitment of this network correlated with psychophysical olfactory performances [32]. Olfactory stimulation recruited additional cerebellar and occipital networks, in which activation also correlated with psychophysical olfactory performances [32]. The recruitment of the cerebellar network was explained by the relation between sniffing and smelling, since the cerebellum is part of the olfactomotor system which is involved in the control of smelling. The recruitment of the occipital network could underline the interconnection between the visual and the olfactory networks or might be a consequence of subjects attempting to visualize the odor source.
Resting-State FC (rsFC) Studies
rsFC studies also confirmed a functional impairment in OD patients. A study in patients with post-traumatic OD [33] revealed different patterns of rsFC in the olfactory network, in comparison to normosmic controls. Particularly, intra-cluster rsFC at the level of the insular cortex and anterior cingulate/frontal cortex was decreased in patients. Inter-anatomical-cluster rsFC (across the piriform cortex, insular cortex, prefrontal cortex, and anterior cingulate/frontal cortex) was increased in patients. The same study also revealed increased rsFC in other brain regions, notably among the thalamic and sensory networks (visual and somatomotor hand area). The author suggested that patients with post-traumatic OD may recruit compensatory mechanisms involving other brain areas [33]. A recent study compared patients with SARS-CoV-2 infection (anosmia, hyposmia, normosmia) to controls without a history of COVID-19 [34]. It was found that the strength of the anterior piriform cortex rsFC was significantly higher in patients. No correlation was found between rsFC values and psychophysical olfactory performance in the patient group [34]. Interestingly, a resting-state fMRI study in patients with congenital anosmia found no difference in rsFC within the olfactory cortex in comparison to healthy controls [35].
A recent study evaluated the effects of olfactory training on FC in patients with post-viral OD [36]. Before the onset of olfactory training, patients had increased rsFC within the visual cortex compared to normosmic controls, suggesting heightened visual cues to compensate for the olfactory deficit. After 3 months of olfactory training, rcFC within the visual cortex was decreased, whereas rsFC between olfactory-related brain regions had increased [36]. This suggests that improvement in olfactory function increases rsFC in the olfactory system. Altogether, these results could also suggest that there is an association between smell and vision during olfactory training.
Is fMRI a Valuable Clinical Tool?
fMRI has been proposed not only as a mean to analyze central olfactory processes in OD patients, but also as a clinical objective tool to evaluate the olfactory function and diagnose OD.
As mentioned above, studies performed at a group level have reported reduced brain activation to olfactory stimulation in OD patients, compared to normosmic controls. Therefore, it is very tempting to consider fMRI as a potential objective diagnostic tool in the clinic. In order to be considered a reliable clinical diagnostic tool, fMRI must fulfill the following criteria: having a good signal-to-noise ratio and having a low interindividual variability. Considering the signal-to-noise ratio, it has been shown that the olfactory BOLD signal can be affected by various physiological or methodological factors such as respiration, magnetic susceptibility artifacts due to air/tissue interface at the skull base, metabolic status or the length of odorous stimulation [1••, 37]. Moreover, it is well known that prolonged exposition to odors induces adaptation, leading to decreased BOLD signal in olfactory areas [38]. To overcome these issues, efforts have been undertaken to improve fMRI’s poorer signal-to-noise ratio in olfactory brain areas. This was accomplished by optimizing olfactory stimulus characteristics (duration of the stimulus, odor intensity, hedonicity, and familiarity) and by using short stimulation periods to avoid habituation [37, 39]. In this vein, it was notably shown that short repetition time and short stimulation length led to stronger BOLD signal increases and shorter time-to-peak responses [37].
Using fMRI as a clinical diagnostic tool would also require that it is possible to differentiate one single OD patient from normosmic controls. For that purpose, the prerequisite is that olfactory stimulation elicits reliable activation patterns between and within subjects and that group-level effects (i.e., normosmia vs. hyposmia) can be generalizable at an individual level. In other words, fMRI must show a low inter-individual variability in the BOLD response pattern. However, even in normosmic subjects, olfactory fMRI has a high inter-individual variability [40]. A recent study aimed to directly investigate the question of the potential clinical usefulness of olfactory fMRI evaluated at the individual patient level [1••]. Results showed that at the group level (normosmic vs. OD), OD patients had significantly lower odor-induced activation in primary and secondary olfactory areas. At the individual level, 94% of the normosmic controls vs. 41% of OD patients activated clusters in the primary olfactory cortex. Based on the % BOLD signal change from the primary olfactory area, orbitofrontal and insular cortices, ROC curve analyses failed to reliably discriminate between OD patients and controls. Of note, no association was found between individual fMRI parameters and psychophysical test scores. The authors concluded that, up to date, the diagnosis of OD on an individual level cannot be done based on fMRI [1••].
Conclusion
Functional MRI constitutes a non-invasive, attractive, and promising method to objectively assess olfactory function. This technique has undoubtedly brought valuable knowledge regarding olfactory processing. Notably, fMRI has allowed the identification of brain regions involved in normal olfactory processing and furthered insights into the impact of olfactory dysfunction on this processing. On a group level, odor-induced brain activation is significantly impaired in patients with OD, suggesting a potential usefulness of fMRI as an objective tool in clinic. However, due to the high inter-individual variability of olfactory fMRI it is not yet realistic to consider it as a clinical diagnostic tool for OD. Although recent studies have brought interesting insights into optimization of stimulation paradigm, further studies are needed to define the most optimal stimulation paradigms and analysis methods before fMRI can be implemented as a routine diagnostic tool for OD.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Yunpeng Z, Han P, Joshi A, Hummel T. Individual variability of olfactory fMRI in normosmia and olfactory dysfunction. Eur Arch Otorhinolaryngol. 2021;278(2):379–87. This study demonstrates the presence of large inter-individual variabilities when using functional MRI among normosmic patients and patients with OD.
Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11(9):628–41.
Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20(2):713–28.
Savic I. Brain imaging studies of the functional organization of human olfaction. Neuroscientist. 2002;8(3):204–11.
Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. Neuroimage. 2006;31(1):386–96.
Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94(8):4119–24.
Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, et al. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13(3):506–19.
Levy LM, Henkin RI, Hutter A, Lin CS, Schellinger D. Mapping brain activation to odorants in patients with smell loss by functional MRI. J Comput Assist Tomogr. 1998;22(1):96–103.
Pellegrino R, Hahner A, Bojanowski V, Hummel C, Gerber J, Hummel T. Olfactory function in patients with hyposmia compared to healthy subjects - an fMRI study. Rhinology. 2016;54(4):374–81.
Han P, Winkler N, Hummel C, Hahner A, Gerber J, Hummel T. Impaired brain response to odors in patients with varied severity of olfactory loss after traumatic brain injury. J Neurol. 2018;265(10):2322–32.
Moon WJ, Park M, Hwang M, Kim JK. Functional MRI as an objective measure of olfaction deficit in patients with traumatic anosmia. AJNR Am J Neuroradiol. 2018;39(12):2320–5.
• Pellegrino R, Farruggia MC, Small DM, Veldhuizen MG. Post-traumatic olfactory loss and brain response beyond olfactory cortex. Sci Rep. 2021;11(1):4043. In this study, the authors were able to discriminate between anosmia or normosmia by analyzing connectivity within a network involving non-olfactory brain regions.
Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci. 2005;25(39):8903–7.
Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–76.
Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–96.
Bird CM, Keidel JL, Ing LP, Horner AJ, Burgess N. Consolidation of complex events via reinstatement in posterior cingulate cortex. J Neurosci. 2015;35(43):14426–34.
Reden J, Maroldt H, Fritz A, Zahnert T, Hummel T. A study on the prognostic significance of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 2007;264(2):139–44.
Pellegrino R, Mainland JD, Kelly CE, Parker JK, Hummel T. Prevalence and correlates of parosmia and phantosmia among smell disorders. Chem Senses. 2021;46.
Iannilli E, Leopold DA, Hornung DE, Hummel T. Advances in understanding parosmia: an fMRI study. ORL J Otorhinolaryngol Relat Spec. 2019;81(4):185–92.
Kipps CM, Duggins AJ, McCusker EA, Calder AJ. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J Cogn Neurosci. 2007;19(7):1206–17.
Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. J Neurosci. 2007;27(11):2908–17.
Boyle JA, Heinke M, Gerber J, Frasnelli J, Hummel T. Cerebral activation to intranasal chemosensory trigeminal stimulation. Chem Senses. 2007;32(4):343–53.
Frasnelli J, Schuster B, Hummel T. Olfactory dysfunction affects thresholds to trigeminal chemosensory sensations. Neurosci Lett. 2010;468(3):259–63.
Huart C, Hummel T, Kaehling C, Konstantinidis I, Hox V, Mouraux A, et al. Development of a new psychophysical method to assess intranasal trigeminal chemosensory function. Rhinology. 2019;57(5):375–84.
Migneault-Bouchard C, Hsieh JW, Hugentobler M, Frasnelli J, Landis BN. Chemosensory decrease in different forms of olfactory dysfunction. J Neurol. 2020;267(1):138–43.
Frasnelli J, Schuster B, Hummel T. Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex. 2007;17(10):2268–75.
Iannilli E, Gerber J, Frasnelli J, Hummel T. Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res. 2007;1139:235–44.
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53.
van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519–34.
Kollndorfer K, Fischmeister FP, Kowalczyk K, Hoche E, Mueller CA, Trattnig S, et al. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. Neuroimage Clin. 2015;9:401–10.
Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta-analysis. Rhinology. 2017;55(1):17–26.
Reichert JL, Postma EM, Smeets PAM, Boek WM, de Graaf K, Schopf V, et al. Severity of olfactory deficits is reflected in functional brain networks-An fMRI study. Hum Brain Mapp. 2018;39(8):3166–77.
Park M, Chung J, Kim JK, Jeong Y, Moon WJ. Altered functional brain networks in patients with traumatic anosmia: resting-state functional MRI based on graph theoretical analysis. Korean J Radiol. 2019;20(11):1536–45.
Esposito F, Cirillo M, De Micco R, Caiazzo G, Siciliano M, Russo AG, et al. Olfactory loss and brain connectivity after COVID-19. Hum Brain Mapp. 2022;43(5):1548–60.
Peter MG, Fransson P, Martensson G, Postma EM, Nordin LE, Westman E, et al. Normal olfactory functional connectivity despite lifelong absence of olfactory experiences. Cereb Cortex. 2021;31(1):159–68.
Jiramongkolchai P, Jones MS, Peterson A, Lee JJ, Liebendorfer A, Klatt-Cromwell CN, et al. Association of olfactory training with neural connectivity in adults with postviral olfactory dysfunction. JAMA Otolaryngol Head Neck Surg. 2021;147(6):502–9.
Georgiopoulos C, Witt ST, Haller S, Dizdar N, Zachrisson H, Engstrom M, et al. Olfactory fMRI: implications of stimulation length and repetition time. Chem Senses. 2018;43(6):389–98.
Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52(6):1097–108.
Kleinhans NM, Reilly M, Blake M, Greco G, Sweigert J, Davis GE, et al. FMRI correlates of olfactory processing in typically-developing school-aged children. Psychiatry Res Neuroimaging. 2019;283:67–76.
Morrot G, Bonny JM, Lehallier B, Zanca M. fMRI of human olfaction at the individual level: interindividual variability. J Magn Reson Imaging. 2013;37(1):92–100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on RHINOLOGY: Taste and Smell Disorders
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Regemorter, V., Rombaux, P., Dricot, L. et al. Functional Imaging in Olfactory Disorders. Curr Otorhinolaryngol Rep 10, 421–426 (2022). https://doi.org/10.1007/s40136-022-00433-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-022-00433-2