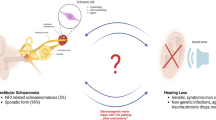
Abstract
Purpose of Review
The aim of this review is to highlight relevant literature on the role of tumor necrosis factor alpha (TNFα) in sensorineural hearing loss (SNHL) and vestibular schwannomas (VS).
Recent Findings
A comprehensive review of publically available databases including PubMed was performed. The mechanism by which hearing loss occurs in VS is still unknown and likely multifactorial. Genetic differences between VSs and tumor-secreted proteins may be responsible, at least in part, for VS-associated SNHL. TNFα has pleotropic roles in promoting inflammation, maintaining cellular homeostasis, inducing apoptosis, and mediating ototoxicity in patients with sporadic VS. TNFα-targeted therapies have shown efficacy in both animal models of sensorineural hearing loss and clinical trials in patients with immune-mediated hearing loss. Efforts are underway to develop nanotechnology-based methods to target TNFα and other pathogenic molecules in VS.
Summary
Development of molecularly targeted therapies against TNFα represents an important area of research in ameliorating VS-associated hearing loss.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Mahaley MS, Mettlin C, Natarajan N, Laws ER, Peace BB. Analysis of patterns of care of brain tumor patients in the United States: a study of the brain tumor section of the AANS and the CNS and the Commission on Cancer of the ACS. Clin Neurosurg. 1990;36:347–52.
Thakur JD, Banerjee AD, Khan IS, Sonig A, Shorter CD, Gardner GL, et al. An update on unilateral sporadic small vestibular schwannoma. Neurosurg Focus. 2012;33(3):E1. https://doi.org/10.3171/2012.6.FOCUS12144.
Jacob A, Oblinger J, Bush ML, et al. Preclinical validation of AR42, a novel histone deacetylase inhibitor, as treatment for vestibular schwannomas. Laryngoscope. 2012;122:174–89.
Goutagny S, Raymond E, Esposito-Farese M, Trunet S, Mawrin C, Bernardeschi D, et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neuro-Oncol. 2015;122(2):313–20. https://doi.org/10.1007/s11060-014-1710-0.
Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230(4728):943–5. https://doi.org/10.1126/science.3933111.
Braumüller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5.
Zhao X, Rong L, Zhao X, Rong L, Zhao X, Li X, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122(11):4094–104. https://doi.org/10.1172/JCI64115.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. https://doi.org/10.1038/nrc3611.
Roosli C, Linthicum FH, Cureoglu S, Merchant SN. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: an underappreciated entity. Otol Neurotol. 2012;33(3):473–80. https://doi.org/10.1097/MAO.0b013e318248ee02.
Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40(1):10.
(1996) The early history of the neurofibromatoses: evolution of the concept of neurofibromatosis type 2. Arch Otolaryngol Head Neck Surg 122:1240–1249.
Nadol JB, Diamond PF, Thornton AR. Correlation of hearing loss and radiologic dimensions of vestibular schwannomas (acoustic neuromas). Am J Otol. 1996;17(2):312–6.
Caye-Thomasen P, Dethloff T, Hansen S, Stangerup S-E, Thomsen J. Hearing in patients with intracanalicular vestibular schwannomas. Audiol Neurotol. 2007;12, 12(1)
Gouveris HT, Victor A, Mann WJ. Cochlear origin of early hearing loss in vestibular schwannoma. Laryngoscope. 2007;117:680–3.
Stankovic KM, Mrugala MM, Martuza RL, Silver M, Betensky RA, Nadol JB, et al. Genetic determinants of hearing loss associated with vestibular schwannomas. Otol Neurotol. 2009;30(5):661–7. https://doi.org/10.1097/MAO.0b013e3181a66ece.
Lassaletta L, Martínez-Glez V, Torres-Martín M, Rey JA, Gavilán J. cDNA microarray expression profile in vestibular schwannoma: correlation with clinical and radiological features. Cancer Genet Cytogenet. 2009;194(2):125–7. https://doi.org/10.1016/j.cancergencyto.2009.06.016.
Silverstein H. A rapid protein test for acoustic neurinoma. Arch Otolaryngol. 1972;95(3):202–4. https://doi.org/10.1001/archotol.1972.00770080344003.
Silverstein H. Labyrinthine tap as a diagnostic test for acoustic neurinoma. Otolaryngol Clin N Am. 1973;6(1):229–44.
Schmitt HA, Pich A, Schröder A, Scheper V, Lilli G, Reuter G, et al. Proteome analysis of human perilymph using an intraoperative sampling method. J Proteome Res. 2017;16(5):1911–23. https://doi.org/10.1021/acs.jproteome.6b00986.
Lysaght AC, Kao S-Y, Paulo JA, Merchant SN, Steen H, Stankovic KM. Proteome of human perilymph. J Proteome Res. 2011;10(9):3845–51. https://doi.org/10.1021/pr200346q.
Ji H, Cao R, Yang Y, et al. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun. 2014;5:4944.
Brieger J, Bedavanija A, Lehr H-A, Maurer J, Mann WJ. Expression of angiogenic growth factors in acoustic neurinoma. Acta Otolaryngol. 2016;123:1040–5.
• Plotkin SR, Stemmer-Rachamimov AO, Barker FG, Halpin C, Padera TP, Tyrrell A, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–67. First demonstration that VEGF blockade can improve hearing in patients with NF2 vestibular schwannomas.
Plotkin SR, Merker VL, Halpin C, Jennings D, McKenna MJ, Harris GJ, et al. Bevacizumab for progressive vestibular schwannoma in neurofibromatosis type 2: a retrospective review of 31 patients. Otol Neurotol. 2012;33(6):1046–52. https://doi.org/10.1097/MAO.0b013e31825e73f5.
Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72(9):3666–70. https://doi.org/10.1073/pnas.72.9.3666.
Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312(5996):724–9. https://doi.org/10.1038/312724a0.
Gray PW, Aggarwal BB, Benton CV, Bringman TS, Henzel WJ, Jarrett JA, et al. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984;312(5996):721–4. https://doi.org/10.1038/312721a0.
Aggarwal BB, Kohr WJ, Hass PE, Moffat B, Spencer SA, Henzel WJ, et al. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985;260(4):2345–54.
Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22. https://doi.org/10.1016/j.neuroscience.2015.06.038.
Papathanasiou S, Rickelt S, Soriano ME, Schips TG, Maier HJ, Davos CH, et al. Tumor necrosis factor-α confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med. 2015;21(9):1076–84. https://doi.org/10.1038/nm.3925.
Zhao S, Yin KW, Goodson NJ. FRI0134 association between vitamin D deficiency and markers of disease activity in axial spondyloarthritis: table 1. Ann Rheum Dis. 2014;73(Suppl 2):430.2–430. https://doi.org/10.1136/annrheumdis-2014-eular.2134.
Zou J, Pyykko I, Sutinen P, Toppila E. Vibration induced hearing loss in guinea pig cochlea: expression of TNF-alpha and VEGF. Hear Res. 2005;202(1-2):13–20. https://doi.org/10.1016/j.heares.2004.10.008.
Riva C, Donadieu E, Magnan J, Lavieille J-P. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp Gerontol. 2007;42(4):327–36. https://doi.org/10.1016/j.exger.2006.10.014.
Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope. 2002;112(9):1627–34. https://doi.org/10.1097/00005537-200209000-00019.
Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83(4):575–83. https://doi.org/10.1002/jnr.20764.
MacArthur CJ, Pillers DA, Pang J, Kempton JB, Trune DR. Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope. 2011;121(2):365–71. https://doi.org/10.1002/lary.21349.
Trune DR, Larrain BE, Hausman FA, Kempton JB, MacArthur CJ. Simultaneous measurement of multiple ear proteins with multiplex ELISA assays. Hear Res. 2011;275(1-2):1–7. https://doi.org/10.1016/j.heares.2010.11.009.
Perny M, Roccio M, Grandgirard D, Solyga M, Senn P, Leib SL. The severity of infection determines the localization of damage and extent of sensorineural hearing loss in experimental pneumococcal meningitis. J Neurosci. 2016;36(29):7740–9. https://doi.org/10.1523/JNEUROSCI.0554-16.2016.
So H, Kim H, Lee JH, et al. Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-kappaB. J Assoc Res Otolaryngol. 2007;8(3):338–55. https://doi.org/10.1007/s10162-007-0084-9.
Kim HJ, Oh GS, Lee JH, Lyu AR, Ji HM, Lee SH, et al. Cisplatin ototoxicity involves cytokines and STAT6 signaling network. Cell Res. 2011;21(6):944–56. https://doi.org/10.1038/cr.2011.27.
Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis. 2011;2(7):e180. https://doi.org/10.1038/cddis.2011.63.
Hoang KN, Dinh CT, Bas E, Chen S, Eshraghi AA, Van De Water TR. Dexamethasone treatment of naive organ of Corti explants alters the expression pattern of apoptosis-related genes. Brain Res. 2009;1301:1–8.
Bas E, Van De Water TR, Gupta C, Dinh J, Vu L, Martinez-Soriano F, et al. Efficacy of three drugs for protecting against gentamicin-induced hair cell and hearing losses. Br J Pharmacol. 2012;166(6):1888–904. https://doi.org/10.1111/j.1476-5381.2012.01890.x.
Pasparakis M. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–411. https://doi.org/10.1084/jem.184.4.1397.
Erickson SL, de Sauvage FJ, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, et al. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372(6506):560–3. https://doi.org/10.1038/372560a0.
Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73(3):457–67. https://doi.org/10.1016/0092-8674(93)90134-C.
Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to IMF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364(6440):798–802. https://doi.org/10.1038/364798a0.
Yang S, Zhang LS, Gibboni R, Weiner B, Bao S. Impaired development and competitive refinement of the cortical frequency map in tumor necrosis factor-deficient mice. Cereb Cortex. 2014;24(7):1956–65. https://doi.org/10.1093/cercor/bht053.
Oishi N, Chen J, Zheng H-W, Hill K, Schacht J, Sha S-H. Tumor necrosis factor-alpha-mutant mice exhibit high frequency hearing loss. J Assoc Res Otolaryngol. 2013;14(6):801–11. https://doi.org/10.1007/s10162-013-0410-3.
Sharaf K, Ihler F, Bertlich M, Reichel CA, Berghaus A, Canis M. Tumor necrosis factor-induced decrease of Cochlear blood flow can be reversed by etanercept or JTE-013. Otol Neurotol. 2016;37:e203–8.
Ihler F, Sharaf K, Bertlich M, Strieth S, Reichel CA, Berghaus A, et al. Etanercept prevents decrease of cochlear blood flow dose-dependently caused by tumor necrosis factor alpha. Ann Otol Rhinol Laryngol. 2013;122(7):468–73. https://doi.org/10.1177/000348941312200711.
Arpornchayanon W, Canis M, Ihler F, Settevendemie C, Strieth S. TNF-alpha inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo. Int J Audiol. 2013;52:545–52.
Ihler F, Pelz S, Coors M, Matthias C, Canis M. Application of a TNF-alpha-inhibitor into the scala tympany after cochlear electrode insertion trauma in guinea pigs: preliminary audiologic results. Int J Audiol. 2014;53:810–6.
Lobo D, García-Berrocal JR, Trinidad A, Verdaguer JM, Ramírez-Camacho R. Review of the biological agents used for immune-mediated inner ear disease. Acta Otorrinolaringologica (English Edition). 2013;64(3):223–9. https://doi.org/10.1016/j.otoeng.2013.06.005.
Matteson EL, Tirzaman O, Kasperbauer J, Facer GW, Beatty CW, Fabry DA, et al. Use of methotrexate for autoimmune hearing loss. Ann Otol Rhinol Laryngol. 2016;109:710–4.
McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88(5):585–9. https://doi.org/10.1177/000348947908800501.
Demirhan E, Eskut NP, Zorlu Y, Cukurova I, Tuna G, Kirkali FG. Blood levels of TNF-α, IL-10, and IL-12 in idiopathic sudden sensorineural hearing loss. Laryngoscope. 2013;123(7):1778–81. https://doi.org/10.1002/lary.23907.
Scherer EQ, Yang J, Canis M, et al. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618–24.
Haubner F, Martin L, Steffens T, Strutz J, Kleinjung T. The role of soluble adhesion molecules and cytokines in sudden sensorineural hearing loss. YMHN. 2011;144:575–80.
Derebery MJ, Rao VS, Siglock TJ, Linthicum FH, Nelson RA. Menière’s disease: an immune complex-mediated illness? Laryngoscope. 1991;101(3):225–9. https://doi.org/10.1288/00005537-199103000-00001.
Nacci A, Dallan I, Monzani F, Dardano A, Migliorini P, Riente L, et al. Elevated antithyroid peroxidase and antinuclear autoantibody titers in Ménière’s disease patients: more than a chance association? Audiol Neurootol. 2010;15(1):1–6. https://doi.org/10.1159/000218357.
Gazquez I, Soto-Varela A, Aran I, Santos S, Batuecas A, Trinidad G, et al. High prevalence of systemic autoimmune diseases in patients with Meniere’s disease. PLoS One. 2011;6(10):e26759. https://doi.org/10.1371/journal.pone.0026759.
• Dilwali S, Landegger LD, Soares VY, Deschler DG, Stankovic KM. Secreted factors from human vestibular schwannomas can cause Cochlear damage. Sci Rep. 2015;5:18599. First demonstration that VS secretions rich in TNFα can cause direct cochlear damage.
van Wijk F, Staecker H, Keithley E, Lefebvre PP. Local perfusion of the tumor necrosis factor α blocker infliximab to the inner ear improves autoimmune neurosensory hearing loss. Audiol Neurotol. 2006;11(6):357–65. https://doi.org/10.1159/000095897.
Derebery MJ, Fisher LM, Voelker CCJ, Calzada A. An open label study to evaluate the safety and efficacy of intratympanic golimumab therapy in patients with autoimmune inner ear disease. Otol Neurotol. 2014;35(9):1515–21. https://doi.org/10.1097/MAO.0000000000000566.
Gazeau P, Saraux A, Devauchelle-Pensec V, Cornec D. Long-term efficacy of infliximab in autoimmune sensorineural hearing loss associated with rheumatoid arthritis. Rheumatology. 2014;53(9):1715–6. https://doi.org/10.1093/rheumatology/keu025.
Rahman MU, Poe DS, Choi HK. Etanercept therapy for immune-mediated cochleovestibular disorders: preliminary results in a pilot study. Otol Neurotol. 2001;22(5):619–24. https://doi.org/10.1097/00129492-200109000-00010.
Matteson EL, Choi HK, Poe DS, Wise C, Lowe VJ, Mcdonald TJ, et al. Etanercept therapy for immune-mediated cochleovestibular disorders: a multi-center, open-label, pilot study. Arthritis Rheum. 2005;53(3):337–42. https://doi.org/10.1002/art.21179.
• Cohen S, Shoup A, Weisman MH, Harris J. Etanercept treatment for autoimmune inner ear disease: results of a pilot placebo-controlled study. Otol Neurotol. 2005;26(5):903–7 Randomized controlled trial of etanercept treatment in patients with AIED. https://doi.org/10.1097/01.mao.0000185082.28598.87.
Taurone S, Bianchi E, Attanasio G, et al. Immunohistochemical profile of cytokines and growth factors expressed in vestibular schwannoma and in normal vestibular nerve tissue. Mol Med Rep. 2015;12:737–45.
• Ren Y, Sagers JE, Landegger LD, Bhatia SN, Stankovic KM. Tumor-penetrating delivery of siRNA against TNFα to human vestibular schwannomas. Sci Rep. 2017;7:12922. First demonstration that tumor-targeted nanotechnology and RNA interference can be leveraged synergistically to mitigate the secretion of ototoxic molecules including TNFα.
Agnihotri S, Jalali S, Wilson MR, et al. The genomic landscape of schwannoma. Nat Genet. 2016;48:1339–48.
Jobin C, Morteau O, Han DS, Balfour Sartor R. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95(4):537–43. https://doi.org/10.1046/j.1365-2567.1998.00646.x.
Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Investig. 1995;95:1669–75.
Dilwali S, Briët MC, Kao S-Y, Fujita T, Landegger LD, Platt MP, et al. Preclinical validation of anti-nuclear factor-kappa B therapy to inhibit human vestibular schwannoma growth. Mol Oncol. 2015;9(7):1359–70. https://doi.org/10.1016/j.molonc.2015.03.009.
Dilwali S, Kao S-Y, Fujita T, Landegger LD, Stankovic KM. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl Res. 2015;166(1):1–11. https://doi.org/10.1016/j.trsl.2014.12.007.
Kandathil CK, Dilwali S, Wu C-C, Ibrahimov M, McKenna MJ, Lee H, et al. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol Neurotol. 2014;35(2):353–7. https://doi.org/10.1097/MAO.0000000000000189.
Stankovic KM (2017) Study of aspirin in patients with vestibular schwannoma. In: https://clinicaltrials.gov/ct2/show/NCT03079999?term=stankovic&rank=1. ClinigalTrials.gov Identifier: NCT03079999. Accessed 30 Nov 2017.
Hellgren K, Smedby KE, Feltelius N, Baecklund E, Askling J. Do rheumatoid arthritis and lymphoma share risk factors?: a comparison of lymphoma and cancer risks before and after diagnosis of rheumatoid arthritis. Arthritis Rheum. 2010;62(5):1252–8. https://doi.org/10.1002/art.27402.
Hellgren K, Smedby KE, Backlin C, Sundstrom C, Feltelius N, Eriksson JK, et al. Ankylosing spondylitis, psoriatic arthritis, and risk of malignant lymphoma: a cohort study based on nationwide prospectively recorded data from Sweden. Arthritis Rheumatol. 2014;66(5):1282–90. https://doi.org/10.1002/art.38339.
Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701.
Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda APM. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72(4):517–24. https://doi.org/10.1136/annrheumdis-2011-201244.
Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56(5):1433–9. https://doi.org/10.1002/art.22579.
Ramiro S, Sepriano A, Chatzidionysiou K, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis. 2017;76:1101–36.
Funding
This work was supported by the CORE grant from American Academy of Otolaryngology—Head and Neck Surgery and the New England Otolaryngological Society research grant (Y.R.), NIH/NIDCD grant R01DC015824 (K.M.S.), the Bertarelli Foundation (K.M.S.), Nancy Sayles Day Foundation (K.M.S.), and the Lauer Tinnitus Research Center (K.M.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Otology
Rights and permissions
About this article
Cite this article
Ren, Y., Stankovic, K.M. The Role of Tumor Necrosis Factor Alpha (TNFα) in Hearing Loss and Vestibular Schwannomas. Curr Otorhinolaryngol Rep 6, 15–23 (2018). https://doi.org/10.1007/s40136-018-0186-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40136-018-0186-4