Abstract
Purpose of review
To report the visual, clinical, and surgical outcomes of cataract surgery following radiation therapy for uveal melanoma (UM) while reviewing the available literature discussing these outcomes.
Recent Findings
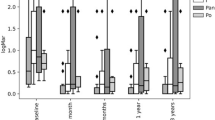
Patients were managed with cataract surgery after radiation for UM at the Bascom Palmer Eye Institute, between January 2014 and February 2021, with a minimum follow-up of 6 months. Only the radiated eye was included. Measured outcomes included BCVA (logMAR), and OCT measurement of macular thickness at 1, 3, 6, and 12 months postoperatively. Paired t-tests were used to compare pre- and postoperative variables. Forty-seven eyes from 47 patients were included. Their mean age was 67.0 ± 11.0 years old; 24 (51%) were male. Preoperatively, 21 (44.6%) had radiation-induced maculopathy, 8 (17%) exudative retinal detachment, and 4 (8.5%) iris synechiae. Cataract types included nuclear sclerosis (80.8%), posterior subcapsular (55.3%), cortical sclerosis (19.1%), and mixed-type (55.3%). Intraoperative events reported were synechialysis (4.2%), floppy iris syndrome (IFIS) (2.1%), sub-tenon’s corticosteroid injections (31.9%), and bevacizumab (21.2%). There were no complications. There was a significant improvement in best corrected visual acuity (BCVA) at the 1st month postoperatively (0.68), and up until the 12th month postoperatively (0.71) compared to baseline (1.14, p < 0.01). OCT measurements of the macular thickness showed a significant increase (363 µm) during the 6th postoperative month compared to baseline (327 µm; p < 0.01).
Summary
Cataract surgery is safe and effective in improving visual acuity in individuals with post-radiation cataracts. Routine macular thickness monitoring should be considered in order to optimize their visual outcome. In the treatment of these patients, a multidisciplinary approach and proper surgical planning are essential, with a focus on early, aggressive anti-inflammatory and anti-VEGF therapy.
Setting
Bascom Palmer Eye Institute, USA.
Design
Retrospective chart review.
Methods
Patients were managed with cataract surgery after radiation for UM at the Bascom Palmer Eye Institute, between January 2014 and February 2021, with a minimum follow-up of 6 months. Only the radiated eye was included. Measured outcomes included BCVA (logMAR) and OCT measurement of macular thickness at 1, 3, 6, and 12 months postoperatively. Paired t-tests were used to compare pre and postoperative variables.
Results
Forty-seven eyes from 47 patients were included. Their mean age was 67.0 ± 11.0 years old; 24 (51%) were male. Preoperatively, 21 (44.6%) had radiation-induced maculopathy, 8 (17%) exudative retinal detachment, and 4 (8.5%) iris synechiae. Cataract types included nuclear sclerosis (80.8%), posterior subcapsular (55.3%), cortical sclerosis (19.1%), and mixed-type (55.3%). Intraoperative events reported were synechialysis (4.2%), floppy iris syndrome (IFIS) (2.1%), sub-tenon’s corticosteroid injections (31.9%), and bevacizumab (21.2%). There were no complications. There was a significant improvement in best corrected visual acuity (BCVA) at the 1st month postoperatively (0.68) and up until the 12th month postoperatively (0.71) compared to baseline (1.14, p < 0.01). OCT measurements of the macular thickness showed a significant increase (363 µm) during the 6th postoperative month compared to baseline (327 µm; p < 0.01).
Conclusion
Cataract surgery is safe and effective in improving visual acuity in individuals with post-radiation cataracts. A multi-disciplinary approach, proper surgical planning, and postoperative anti-inflammatory care are paramount in the management of these individuals.

Similar content being viewed by others
References
Thariat J, Jacob S, Caujolle JP, Maschi C, Baillif S, Angellier G, Mathis T, Rosier L, Carnicer A, Herault J, Salleron J. Cataract avoidance with proton therapy in ocular melanomas. Invest Ophthalmol Vis Sci. 2017;58(12):5378–86.
Aronow ME, Topham AK, Singh AD. Uveal melanoma: 5-year update on incidence, treatment, and survival (SEER 1973–2013). Ocular oncol Pathol. 2018;4(3):145–51.
Stålhammar G. Forty-year prognosis after plaque brachytherapy of uveal melanoma. Sci Rep. 2020;10(1):1–10.
Mathis T, Rosier L, Meniai F, Baillif S, Maschi C, Herault J, Caujolle JP, Kodjikian L, Salleron J, Thariat J. The lens opacities classification system III grading in irradiated uveal melanomas to characterize proton therapy-induced cataracts. Am J Ophthalmol. 2019;201:63–71.
Collaborative Ocular Melanoma Study G. Incidence of cataract and outcomes after cataract surgery in the first 5 years after iodine 125 brachytherapy in the Collaborative Ocular Melanoma Study: COMS Report No. 27. Ophthalmology. 2007;114(7):1363–71.
Ainsbury EA, Barnard S, Bright S, Dalke C, Jarrin M, Kunze S, Tanner R, Dynlacht JR, Quinlan RA, Graw J. Ionizing radiation induced cataracts: recent biological and mechanistic developments and perspectives for future research. Mutat Res/Rev Mutat Res. 2016;770:238–61.
Ainsbury EA, Dalke C, Hamada N, Benadjaoud MA, Chumak V, Ginjaume M, Kok JL, Mancuso M, Sabatier L, Struelens L. Radiation-induced lens opacities: epidemiological, clinical and experimental evidence, methodological issues, research gaps and strategy. Environ Int. 2021;146:106213.
Seals KF, Lee EW, Cagnon CH, Al-Hakim RA, Kee ST. Radiation-induced cataractogenesis: a critical literature review for the interventional radiologist. Cardiovasc Intervent Radiol. 2016;39(2):151–60.
Richardson RB, Ainsbury EA, Prescott CR, Lovicu FJ. Etiology of posterior subcapsular cataracts based on a review of risk factors including aging, diabetes, and ionizing radiation. Int J Radiat Biol. 2020;96(11):1339–61.
Fallico M, Chronopoulos A, Schutz JS, Reibaldi M. Treatment of radiation maculopathy and radiation-induced macular edema: a systematic review. Surv Ophthalmol. 2021;66(3):441–460.
Finger PT, Chin K. Anti–vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol. 2007;125(6):751–6.
Wachtlin J, Bechrakis NE, Schueler AO, Helbig H, Bornfeld N, Foerster MH. Phacoemulsification following treatment of choroidal melanoma. Graefe’s Arch Clin Exp Ophthalmol. 2000;238(12):942–8.
Augsburger JJ, Shields JA. Cataract surgery following cobalt 60 plaque radiotherapy for posterior uveal malignant melanoma. Ophthalmology. 1985;92(6):815–22.
Shields JA. Cataract surgery after radiotherapy for uveal melanoma. Arch Ophthalmol. 1992;110(4):473–4.
Bozkurt TK, Tang Q, Grunstein LL, McCannel TA, Straatsma BR, Miller KM. Outcomes of cataract surgery in eyes with ocular melanoma treated with iodine-125 brachytherapy. J Cataract Refract Surg. 2018;44(3):287–94.
Syed ZA, Pineda R II. Cataract surgery after proton-beam irradiation for uveal tumors. J Cataract Refract Surg. 2017;43(10):1328–34.
Tiew S, Lim C, Sivagnanasithiyar T. Using an excel spreadsheet to convert Snellen visual acuity to LogMAR visual acuity. Eye. 2020;34(11):2148–9.
Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–40.
Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–90.
Koo E, Chang JR, Agrón E, Clemons TE, Sperduto RD, Ferris FL III, Chew EY, Group A-REDSR. Ten-year incidence rates of age-related cataract in the Age-Related Eye Disease Study (AREDS): AREDS report no 33. Ophthalmic Epidemiol. 2013;20(2):71–81.
Klein BE, Klein R, Lee KE. Incidence of age-related cataract: the Beaver Dam Eye Study. Arch Ophthalmol. 1998;116(2):219–25.
Balal S, Jbari AS, Nitiapapand R, Cook E, Akhtar W, Din N, Sharma A. Management and outcomes of the small pupil in cataract surgery: iris hooks, Malyugin ring or phenylephrine?. Eye. 2020;1–5.
Halkiadakis I, Chatziralli I, Drakos E, Katzakis M, Skouriotis S, Patsea E, Mitropoulos P, Kandarakis A. Causes and management of small pupil in patients with cataract. Oman J Ophthalmol. 2017;10(3):220.
Narendran N, Jaycock P, Johnston R, Taylor H, Adams M, Tole D, Asaria R, Galloway P, Sparrow J. The Cataract National Dataset electronic multicentre audit of 55 567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye. 2009;23(1):31–7.
Chan NS-W, Ti S-E, Chee S-P. Decision-making and management of uveitic cataract. Indian J Ophthalmol. 2017;65(12):1329.
Chen JL, Bhat P, Lobo-Chan A-M. Perioperative management of uveitic cataracts. Adv Ophthalmol optom. 2019;4:325.
Horgan N, Shields CL, Mashayekhi A, Teixeira LF, Materin MA, Shields JA. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina. 2008;28(2):263–73.
Singh AD, Pabon S, Aronow ME. Management of radiation maculopathy. Ophthalmic Res. 2012;48(Suppl. 1):26–31.
Monroe AT, Bhandare N, Morris CG, Mendenhall WM. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol* Biol* Phys. 2005;61(3):856–64.
Adhi M, Aziz S, Muhammad K, Adhi MI. Macular thickness by age and gender in healthy eyes using spectral domain optical coherence tomography. PLoS ONE. 2012;7(5):e37638.
Shah NV, Houston SK, Markoe A, Murray TG. Combination therapy with triamcinolone acetonide and bevacizumab for the treatment of severe radiation maculopathy in patients with posterior uveal melanoma. Clin Ophthalmol (Auckland, NZ). 2013;7:1877.
Finger PT, Mukkamala SK. Intravitreal anti-VEGF bevacizumab (Avastin) for external beam-related radiation retinopathy. Eur J Ophthalmol. 2011;21(4):446–51.
Reichstein D. Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol. 2015;26(3):157–66.
Horgan N, Shields CL, Mashayekhi A, Shields JA. Classification and treatment of radiation maculopathy. Curr Opin Ophthalmol. 2010;21(3):233–8.
Kocak N, Saatci AO, Bajin FMS. Combination of photodynamic therapy, intravitreal triamcinolone injection, and standard laser photocoagulation in radiation retinopathy. Ann Ophthalmol. 2006;38(3):243–7.
Horgan N, Shields CL, Mashayekhi A, Salazar PF, Materin MA, O’Regan M, Shields JA. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: a randomized controlled trial. Ophthalmology. 2009;116(7):1383–90.
Kaplan RI, Chaugule SS, Finger PT. Intravitreal triamcinolone acetate for radiation maculopathy recalcitrant to high-dose intravitreal bevacizumab. Br J Ophthalmol. 2017;101(12):1694–8.
Groenewald C, Konstantinidis L, Damato B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye. 2013;27(2):163–71.
Mason JO III, Albert MAJ, Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina. 2007;27(7):903–7.
Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina. 2008;28(7):964–8.
Mashayekhi A, Rojanaporn D, Al-Dahmash S, Shields CL, Shields JA. Monthly intravitreal bevacizumab for macular edema after iodine-125 plaque radiotherapy of uveal melanoma. Eur J Ophthalmol. 2014;24(2):228–34.
Solano JM, Bakri SJ, Pulido JS. Regression of radiation-induced macular edema after systemic bevacizumab. Can J Ophthalmol. 2007;42(5):748–9.
Feng Y, Zhu S, Skiadaresi E, McAlinden C, Tu R, Gao R, Stephens JW, Wang Q, Huang J. Phacoemulsification cataract surgery with prophylactic intravitreal bevacizumab for patients with coexisting diabetic retinopathy: A meta-analysis. Retina (Philadelphia, Pa). 2019;39(9):1720–31.
Rayess N, Mruthyunjaya P. Anti-vascular endothelial growth factor therapy for radiation retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2020;51(4):S44–9.
Schefler AC, Fuller D, Anand R, Fuller T, Moore C, Munoz J, Kim RS, Group RS. Randomized trial of monthly versus as-needed intravitreal ranibizumab for radiation retinopathy–related macular edema: 1-year outcomes. Am J Ophthalmol. 2020;216:165–73.
Fish GE, Jost BF, Snyder WB, Fuller DG, Birch DG. Cataract extraction after brachytherapy for malignant melanoma of the choroid. Ophthalmology. 1991;98(5):619–22.
Gragoudas ES, Egan KM, Arrigg PG, Seddon JM, Glynn RJ, Munzenrider JE. Cataract extraction after proton beam irradiation for malignant melanoma of the eye. Arch Ophthalmol. 1992;110(4):475–9.
Funding
Supported by the NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant (GR004596) to Bascom Palmer Eye Institute and Consejo Nacional de Ciencia y Tecnología CVU810654 (H. Levine).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meeting Presentations: Abstract presented at: Women In Ophthalmology Summer Symposium; August 26–29, 2021; Amelia Island, FL, USA. Abstract presented at: ASCRS annual meeting; April 22–26, 2022; Washington, DC, USA.
This article is part of the Topical Collection on Cataract & Refractive Surgery.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepulveda-Beltran, P.A., Levine, H., Gibbons, A.G. et al. Post-Radiation Cataract Management: Outcomes in Individuals with Uveal Melanoma. Curr Ophthalmol Rep 10, 218–227 (2022). https://doi.org/10.1007/s40135-022-00304-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-022-00304-5