Abstract
Purpose of the Review
This review article aims to show the actual role of Imaging, especially DECT (Dual Energy CT), in recognition of renal calculi.
Recent Findings
CT and in particular DECT have some implications in renal stone disease; CT is considered the gold-standard in the diagnosis in case of acute flank pain caused by nephrolithiasis, better than ultrasound, that represent the first approach, in some specific cases. DECT instead in these days, has increase a very particular role.
Summary
About 12% of the world’s population will experience urinary stones, and 50% of affected people experience a recurrence within 10 years after their first diagnosis. There are many different types of calculi, that could form and stay or could form and then goes to localize in different anatomical site in the urinary system: kidney, ureters, bladder, and urethra. Calculi, especially with high dimensions, cause the typical flank pain, also known as renal colic. The precise cause of their formation is still unknown, it is frequently believed that mineral deposition on a nidus of the mucoprotein matrix is what causes them to form. The preferred Imaging method for detecting urinary stones is ultrasonography (used like the first approach), and Computed Tomography (gold standard), more rapid if “low-dose CT”. In these days, Dual Energy Computed Tomography is useful to determine the composition of the calculation. In fact, it is more effective than single-energy CT; it creates a better separation of stones from iodine; and it allows better measures of stone composition with better differentiation of urate stones from others (even at low doses).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ancient Egyptians first documented renal stone disease (also known as nephrolithiasis or urolithiasis) about 2000 years ago. About 12% of the world’s population will experience urinary stones in their lives [1•, 2].
Males are more likely than females and 50% of affected people experience a recurrence within 10 years after their first diagnosis. The renal calyces and pelvis are where kidney stones develop.
Although their exact cause is uncertain, it is frequently believed that mineral deposition on a nidus of the mucoprotein matrix is what causes them to form.
Family history, metabolic syndrome, hot climates, persistent dehydration, abnormalities of the urinary tract, and recurrent urinary tract infections are risk factors for kidney stone calculi.
The preferred imaging method for detecting urinary stones as a first approach is ultrasonography (US), meanwhile computed tomography (CT), or sometimes better Dual Energy CT (DECT), is the gold standard and is appropriate in some different cases, especially for non-detected ureteric stones.
Anatomy
The urinary system is made up of the kidneys, ureters, urinary bladder, and urethra.
Kidneys produce urine and are responsible for various actions of the urinary system.
Ureters transport urine away from the kidneys to the urinary bladder, which serves as temporary urine storage.
Urethra is a tubular organ that connects the urinary bladder to the outside world.
Kidneys are organs responsible for filtering blood and eliminating waste through urine [3]. Each adult kidney is approximately 3 cm thick, 6 cm wide, and 12 cm long, with an indentation called the hilum. Kidney weights and clinical chemistry parameters show age- and sex-related variations. They are located between the third lumbar and twelfth thoracic vertebrae, one on each side of the spinal column. Kidneys are retroperitoneal, situated behind the parietal peritoneum and against the posterior abdominal wall. The kidney’s parenchyma is composed of the cortex and medulla, with the renal cortex being the outside and the medulla being the inside. The renal medulla is made up of multiple renal pyramids with straight blood vessels and tubular features. The renal pelvis is situated in the renal sinus and connects to the ureter. Nephrons produce urine, which travels through collecting ducts and small calyces. The kidneys’ functions are influenced by the complex process of aging, with the glomerular filtration rate (GFR) steadily declining with normal aging and can be influenced by superimposed diseases. Nephron numbers decrease microscopically when glomerulosclerosis progresses, with derangements in podocyte biology possibly involved [4].
Urine is transported from the renal pelvis to the bladder through a tiny tube, the ureters, about 25 cm long. The ureter is made up of three layers: fibrous connective tissue, inner circular and external longitudinal smooth muscles, and the mucosa.
The urinary bladder is temporarily stored in the pelvic cavity, behind the symphysis pubis, below the parietal peritoneum. The size and shape of the bladder vary depending on the amount of urine it holds and pressure from other organs [5].
The urethra, a thin wall tube, carries urine from the bladder’s floor to the outside. The internal urethral sphincter and external urethral sphincter regulate the urethral flow of urine. The physical anatomy and physiology of the lower urinary tract vary between men and women. In females, the external urethra opens to the outside before the vagina, while in males, the prostatic urethra, membranous urethra, and spongy urethra are the first segments [6].
Pathogenesis of Calculus Formation
The precise cause of the formation of calculi is still unknown; in fact, in some people, minerals depose on a nidus of mucoprotein matrix along the urinary system.
Diet is considered to have a crucial role in urinary stone formation [7], but in general, little is known about the factors that can put a patient at risk for renal calculus formation.
Theories on the genesis of calculi must include the idea of urinary supersaturation. When a solution has more dissolved substance than it can hold, this is known as supersaturation, and it can cause metabolite crystals to precipitate.
There has been evidence that urinary calculus composition and supersaturation values are correlated [8].
The individual metabolites and the patient’s phenotype affect the likelihood of supersaturation. Patients with metabolic disturbances such as gout, renal tubular acidosis, and hypercalciuria are at a higher risk for developing calculus disease or having calculi recur. Furthermore, these patients have an increased probability of recurrent infections of the urinary tract [9].
Clinic and Symptoms
The most usual initial sign of blocking urolithiasis is loud pain, which is typically colicky in nature.
Acute renal colic, in fact, is severe pain resulting from the presence of a stone in the urinary system. The stone can be present anywhere along the path between the kidneys and the urethra.
The colic appears as a severe form of acute flank discomfort that usually begins over the costovertebral angle and extends anteriorly and inferiorly to the groin or testicle. The site of obstruction and the area of pain are related: pain radiating to the testis or labium is related to lower ureteral blockage, while flank pain is related to renal pelvic or proximal ureteral obstruction. As the stone migrates distally and approaches the bladder, the patient may experience dysuria, urinary frequency, urgency, or difficulty in urination.
In fact, urgency and suprapubic pain can result from stones at the ureterovesical junction [10].
There can be also nausea, vomiting, fever, and haematuria. Even in the absence of obstruction, ureteral or renal stones can cause gross or microscopic haematuria. Small calculi may not cause obstruction, but rather a recurrent urinary tract infection [11, 12]. Renal function and hematologic state are evaluated using blood and urine investigations. Elevated levels of electrolytes, blood urea nitrogen, and creatinine are particularly indicative of renal and metabolic health. In reaction to stress or infection, the WBC count may increase, in some chronic calculus disease patients, the hemoglobin level may decrease. Hematuria and pyuria are found during a urine analysis, and urine pH is also measured.
A urine culture in the setting of infection will help with appropriate antibiotic selection.
Diseases with the Same Clinic of Renal Colic
There are different clinical situations that could present like renal colic (flank pain) [13], that the physicians must recognize; in particular:
-
Non-lithiasic diseases: pyelonephritis, abscess, papillary necrosis, kidney infarction, perirenal hematoma, urothelial neoplasms, malformations.
-
Extra-urinary diseases: acute appendicitis, acute diverticulitis visceral twist, bowel obstruction, volvulus, acute cholecystitis, complicated ovarian masses, acute pancreatitis, aortic dissection, aortic rupture, spleen rupture, lymphoma with hemorrhage, retroperitoneal masses, and uterine leiomyomas.
Different Type of Stones
Calcium oxalate is the most prevalent substance in urinary stones [14].
Urinary calculi can be divided into five categories: calcium, magnesium ammonium phosphate, uric acid, cystine and medicines and their metabolites [15] (Table 1).
Calculi Based on Calcium
Between 70 and 80% of urinary tract calculi are calcium-based. The most prevalent calcium-based calculi are calcium oxalate calculi, which account for 60% of all calculi [16].
Hypercalciuria is the main contributor to the formation of calcium-based calculi.
Deficient calcium reabsorption inside the renal tubules leads to renal hypercalciuria. Additionally, increased intestinal calcium absorption might cause hypercalciuria.
Hypocitraturia (which may develop in the presence of chronic diarrhea, distal renal tubular acidosis, and thiazide use), hyperoxaluria, and aberrant uric acid metabolism (with or without primary gout) are other underlying disorders that contribute to calcium-based calculus formation.
A main, secondary, or idiopathic cause of calcium-based calculus development is hyperoxaluria. A rare autosomal recessive condition known as primary hyperoxaluria results in enhanced glyoxylate oxidation to oxalate due to enzymatic faults. More common is secondary hyperoxaluria, which can be seen in the setting of bowel surgery, inflammatory bowel disease, excessive in-take of vitamin C, and renal insufficiency.
Magnesium Ammonium Phospate Calculi
Struvite calculi are about 15–20% of all urinary calculi [17]. These calculi are brought on by urease-producing bacteria such Proteus, Pseudomonas, Klebsiella species, and enterococci that cause urinary tract infections.
The enzyme urease hydrolyses urea into carbon dioxide and ammonia, increasing urinary pH and encouraging the synthesis of calcium carbonate. Then, calcium carbonate and struvite combine to produce enormous calculi that fill the renal collecting system.
A “staghorn calculus” is a struvite calculus that involves the renal pelvis and extends into at least two calyces due to its similarity to a stag’s antler.
Uric Acid Calculi
Urinary calculi containing uric acid account for 5–10% of all urinary calculi. Urine acidity and hyperuricosuria both promote the production of uric acid calculi. Gout and persistent diarrhea are common causes. In comparison to the general population, patients with a high body mass index or diabetes have more acidic urine and a significantly increased propensity to develop uric acid calculi. On radiography, pure uric acid calculi are radiolucent, but they are easily recognizable on a CT scan [18].
Cystine Calculi
The cystine calculi make up between 1 and 3% of all urinary calculi and are primarily brought on by cystinuria, a metabolic condition brought on by a hereditary deficiency in renal transport.
Cystine calculi can be radiolucent and are frequently referred to as “ground-glass” calculi. Certain cystine calculi have been reported to possess empty regions that appear as low-attenuation foci on a CT scan.
Medication-Induced Calculi
The use of some medications for an extended period or in excess might result in medication-induced calculi. Urinary calculi are particularly well-known side effects of indinavir, and other protease inhibitors used to treat HIV. Ephedrine, a stimulant and weight-loss aid, and guaifenesin, an expectorant, are typical herbal supplements that can cause renal calculi. Matrix calculi may develop because of many other drugs and their metabolites.
On a CT scan, some of these calculi, particularly those connected to indinavir, might be radiolucent. Therefore, even if a CT scan is negative, a patient’s medication history and appearance may be sufficient to diagnose a condition.
Preventative measures such as medication modifications, dose adjustments, increased diuresis and perhaps drugs to modulate urine pH are the mainstays of treatment for medication-induced calculi.
Imaging Modalities
Since 1930, the diagnostic approach to urinary tract pathology has been dominated by urography for a long time. Before 1980, 10 million urograms were performed in the USA each year. Today less than 600,000 are performed. All this is due to the introduction in this period of computerized tomographic methods, first of all RT-HD Ultrasound. The CT, to see the calculations, was introduced in 1975 but only with the introduction of the spiral technology (1989) there was the real possibility of the visualization of calculations and dilated urinary tract (Table 2).
In the examination of urinary calculi, several imaging modalities, such as radiography, ultrasonography, CT, and MRI, each has specific advantages and limits. To identify the best appropriate imaging modalities, a personalized strategy should be used, considering stone size, location, patient characteristics, and clinical context. For complete stone examination, CT, particularly non-contrast CT, remains the preferred modality [13].
Ultrasonography
Ultrasound (US) is routinely used as one of the first examinations of the urinary system and is typically successful in detecting calculi.
Ultrasonography is a non-ionizing radiation procedure that can be performed at the patient’s bedside.
Because ultrasonography does not emit ionizing radiation, it is helpful when used on young patients, pregnant patients, or patients who experience recurrent episodes of urolithiasis. Additionally, calculus composition is not a factor in ultrasound since, if they are visible within the field of view (FOV), almost all calculi will exhibit echogenicity and shadowing. Stones in the calyces, pelvis, pyeloureteric, and vesicoureteral junctions can be found with this technique. Despite, the fact that CT is the gold standard diagnostic for ureteric stones, screening patients using ultrasound in the emergency room can help prevent CT in more than half of patients, resulting in a reduced cumulative radiation dose without increasing complications, emergency department visits, or hospitalizations [19].
US can find calculi in the urinary system that are as small as 0.5 mm in size and the sonographic appearance of nephrolithiasis consists of echogenic foci within the renal system with acoustic shadowing due to reflection and absorption of sound by the stone (Fig. 1). It happens when a region of interest has a high concentration of solid tissue (such as bones or stones) at an interface with a high acoustic impedance mismatch (such as soft tissue/air) [20].
For ureteral stones, US has a sensitivity of 45% and specificity of 94%, while for renal stones, it has a sensitivity of 45% and specificity of 88%.
Often, if the stone is small, the shadow cone is not detected, so only the permanence of the hyperechoic image must be sought. To detect the acoustic shadow, the calculation must be larger than 3 mm.
US is also useful for assessing for complications, such as hydronephrosis (dilatation of the urinary collecting system of the kidney) or pyonephrosis (an infection of the kidney with pus in the upper collecting system), seen on ultrasound with dilatation of the pelvicalyceal system, echogenic debris in the collecting system and fluid–fluid levels within the collecting system. Like CT, ureteral dilatation to the level of the calculus and the collecting system can be directly visualized to show obstruction. However, dilatation without blockage can also exist and is challenging to detect. It is helpful to trace the course of the ureter with the probe to assess the ureteral jet and any unilateral reductions, to assess the potential presence of ureteral obstruction [21].
Because of the underlying intestinal gas and the relative depth of the ureter within the pelvis, direct visualization of ureteral calculi with ultrasonography might also be challenging. When obese patients have a lot of intervening fat, ultrasound visualization may become much more difficult.
Color Doppler Ultrasound
Color Low Doppler (CFD) is a further sonographic modality that could be used as a diagnostic aid. Within a user-defined area, Color Doppler enables the viewing of flow direction and velocity. The sonographer designates a region of interest, and the Doppler changes of returning ultrasound waves are color-coded inside according to average velocity and direction.
Color Doppler imaging can be used to elicit a twinkle artifact in the anticipated area of shadowing on gray-scale imaging to corroborate the visualization of a calculus. Behind a reflecting item (like calculus), it happens as a focus of alternate colors on the Doppler signal, giving the impression of turbulent blood flow.
Compared to gray-scale imaging alone, twinkle artifact on Doppler appears to increase the sensitivity of ultrasound for calculi. A substantial positive predictive value (78%) exists between the presence of renal twinkling artifact on sonography and the presence of nephrolithiasis on unenhanced CT [22] (Fig. 2).
The images a and b show hydronephrosis (orange arrow) due to the presence of a stone at the ureterovesical junction. Image c shows a hyperechoic image of about 1 cm, with a posterior shadow cone, at the level of the ureteral outlet in the bladder (orange circle); using the Doppler d it is possible to see the ‘twinkle artifact’ that diagnoses urinary calculus (Color figure online)
Another artifact visualized on CFD, associated with the twinkle artifact, is the color comet tail. The color comet-tail artifact is visible when Color Doppler is used to examine a small, highly reflective (often calcific) item. Twinkle artifact occurs and immediately deep into the object, a tail of a linear aliased band of color extends away from the probe [23].
Another important feature to be evaluated in urolithiasis through the use of Color Doppler US is the ureteral jet.
The ureteral jet represents the movement of urine from the ureter into the urinary bladder. The presence of urinary stones in cases of urolithiasis can induce obstruction or partial blockage of the ureter, resulting in altered or reduced urine flow. The examiner can observe the jet and assess its features, such as velocity, direction, and symmetry, using Color Doppler US. A normal ureteral jet appears within the bladder as a continuous, symmetrical, and laminar flow of color-coded urine [24].
A compromised ureteral jet in urolithiasis can be seen as a turbulent or discontinuous flow pattern using Color Doppler US. The presence of urinary stones blocking the ureter can cause variations in velocity, directionality, or even the entire absence of urine flow. These findings can help with the diagnosis and therapy of urolithiasis by indicating the severity and location of the obstruction [25].
Plain Radiograph
For many years, the initial exam of choice in the evaluation of acutely developing flank pain was a kidney, ureter, and bladder radiograph (KUB radiograph), an AP supine view of the abdomen.
Compared to unenhanced CT, the radiation exposure is quite low.
KUB has a 44–77% sensitivity and specificity range. If NC-CT is being contemplated, it shouldn’t be done.
Many urinary tract calculi are radiopaque (around 90%) [26].
Radiopaque stones:
-
Calcium stones (Calcium oxalate, calcium phosphate, mixed)
Poor radiopaque stones:
-
Struvite stones
-
Cystine stones (radiopaque compared to soft tissue, although less than calcium-containing calculi)
-
Apatite
Radiolucent stones:
-
Uric acid stones
-
Rare types of stones (xanthine, ammonium urate, 2,8-dihydroxyadenine, drug stones)
On radiography, there may be non-specific secondary signs of renal colic. These include perinephric fluid that obscures the renal outline, moderate splinting, and bowel ileus.
Unfortunately, due to the possibility of bowel contents, soft tissues laying on top, gas, and osseous structures obstructing small radiopaque calculi, radiography has been found to be only around 60% sensitive overall in the identification of urolithiasis. Also, indirect signs of nephrolithiasis, like obstruction and hydronephrosis, cannot be adequately assessed [27].
Bladder calculi can be solitary or numerous, and they frequently have a high size. Internal lamination is frequently seen, much like the outer layer of an onion. They only make up 5% of all stones in the urinary system.
It is challenging to spot small ureteral stones on a plain radiograph; if there is a clinical association between flank pain and renal colic, it is crucial to follow the course of the ureter and look for any calcifications [28•].
Urography
Intravenous urography (IVU), also known as intravenous pyelography, provides valuable information regarding the presence, location, size, and obstruction caused by these stones within the urinary tract.
A contrast agent is injected into a patient’s vein, where it is subsequently filtered and eliminated by the kidneys. X-ray images of the kidneys, ureters, and bladder are acquired at various time intervals as the contrast material moves through the urinary system [29].
While IVU has historically been a popular technique for evaluating the urinary tract, its sensitivity, and specificity for detecting urinary calculi are not as good as other imaging modalities such as computed tomography (CT). Smaller stones, particularly those less than 5 mm in diameter, may be overlooked or hardly viewed.
An obstruction produced by urinary calculi can be evaluated with IVU. The contrast material emphasizes the urinary system, allowing for the identification and characterization of any ureteral obstructions or constrictions.
Furthermore, IVU allows for the evaluation of renal function. The excretion of contrast material shows the renal function and urinary system patency. IVU determines the influence of urinary calculi on kidney healing by measuring renal function [30].
While IVU has various advantages, there are certain things to consider. Ionizing radiation is used in the operation, which may limit its use in some patient populations, such as pregnant women and children. Furthermore, IVU focuses primarily on imaging and localizing urinary calculi, providing little information about the composition and nature of the stones.
Finally, intravenous urography is a useful imaging tool for studying urinary calculi. However, while contemplating its application, the possible dangers connected with radiation exposure and the restricted stone characterization capabilities of IVU should be considered [31].
Unenhanced CT
Since its initial usage for this indication in the late 1990s, CT rapidly became the modality of choice in the examination of suspected urolithiasis.
CT KUB (computed tomography of the kidneys, ureters, and bladder) is a non-invasive approach for diagnosing urolithiasis. In an emergency context, it is usually considered the first imaging modality for suspected urolithiasis. Unenhanced CT performed in the emergency room for the evaluation of urolithiasis accounts for slightly more than 20% of all CT exams performed for the evaluation of acute abdominal discomfort [32].
Non-contrast CT scans have a reported sensitivity and specificity of 95% or higher for detecting urinary calculi.
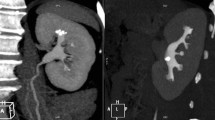
They generate high-resolution images that allow for the exact detection of stones as small as 1–2 mm. These scans can detect the presence, size, location, and shape of urinary stones. Non-contrast CT scans are especially useful in spotting probable urinary stone problems. It can reveal symptoms of obstruction, such as hydronephrosis or hydroureter. They can also detect any related illnesses or abnormalities that may necessitate further medical attention (Fig. 3).
Images a and b refer to the same patient; in the first, dilatation of the right renal pelvis can be observed (orange arrow) due to the presence of calculus at the level of the right uretero-vesical junction (orange circle). Image c shows a stone in the left renal pelvis (red circle) (Color figure online)
The acquisition of images takes only seconds with modern equipment, resulting in more efficient imaging evaluation. Examinations can also be anatomically tailored when the location of calculus is known [33].
A non-contrast CT scan can detect 99% of renal tract calculi but vary considerably in density:
-
Calcium oxalate ± calcium phosphate: 400–800 HU
-
Pure calcium phosphate: 400–800 HU
-
Struvite: 400–600 HU
-
Uric acid: 100–200 HU
-
Cystine: like uric acid stones 100–200 HU
Two radiolucent stones are worth mentioning:
-
Medication (protease inhibitor (indinavir)) stones
-
Pure matrix stones [34].
An important sign on the non-contrast CT to evaluate the presence of a urinary calculus is called the “Rim Sign”, this sign is more cranial to the site where the stone has its largest transversal diameter, this is because at this level the ureteral wall is distended from the calculus and therefore it is not able to produce the rim sign, while at the level of the upper pole of the stone, the calculus with a calcific consistency can be observed and externally a surrounding soft tissue rim, representing the oedematous ureteric wall. The rim sign is important for the differential diagnosis with other pathologies, such as vascular calcifications, which form an incomplete calcific ring along the vessel walls, or phleboliths that usually have invisible walls [35].
In conclusion, non-contrast CT scans are a common and useful tool in the research of urinary stones. They provide detailed images that aid in the diagnosis, evaluation, and localization of urinary tract stones [36].
CT Intravenous Urography
CTU (or CT IVU), also known as CT intravenous pyelography (CT IVP), has displaced standard IVU in imaging the genitourinary tract. It involves the administration of a contrast agent, typically iodine-based, intravenously, which helps enhance the visualization of the urinary tract during the CT scan [37].
By capturing images of the contrast agent passing through the urinary system, CT IVU can precisely localize stones within the kidneys, ureters, or bladder.
CTU has high sensitivity (96–100%) and high specificity (94–100%) for identifying ureteric and bladder calculi.
It provides both anatomical and functional information but at a higher radiation exposure [38].
The CTU acquisition protocol, which is a multi-phase acquisition, is used as the basis for the DECT acquisition approach as demonstrated by the paragraph that follow.
MRI
Although not as often utilized as CT scans, magnetic resonance imaging is a non-invasive imaging technique that can be utilized for assessing urinary stones [39].
On traditional MR imaging, stones appear as nonspecific signal voids that are easily dismissed or confused with other structures or abnormalities. As a result of this limitation, guidelines limit the use of MRI as a kidney stone imaging modality, and radiologists no longer attempt to identify stones based on MRI images.
MRI, like ultrasound, does not require ionizing radiation and can be used alone or in conjunction with radiography to evaluate pediatric, pregnant, or serially scanned patients.
MRI can provide important details about the surrounding tissues and organs, assisting in the identification of any relevant abnormalities or disorders [40].
Depending on their composition and location, urinary stones can appear as areas of signal void or distortion on MRI scans. They can be detected and located using a variety of MRI sequences. T2-weighted sequences are especially good for identifying stones because the fluids are hyperintense and make the calcifications more visible. On a T1-weighted MRI sequence the stone is displayed as a hypointense signal of void, which is difficult to visualize (Fig. 4).
In detecting very small urinary stones or stones with low density, MRI may not be as sensitive as CT scans.
MRIs are often more time-consuming and may need the patient to remain motionless for a longer period of time than CT scans. MRI scanner availability and experience in performing and interpreting MRI tests for urinary stones can differ depending on the healthcare facility.
A contrast agent may or may not be utilized in MRI examinations of urinary stones, depending on the clinical circumstances and the individual requirements. Contrast-enhanced MRI can aid in the visualization of certain structures and in identifying urolithiasis-related complications such as infection or blockage [41].
It is worth mentioning an RM technique that is frequently used for the investigation of the urinary system, which is RM-Urography. MR-Urography is a developing method that has the potential to deliver the most comprehensive and specific imaging diagnostic available for numerous urinary tract problems noninvasively and without the use of ionizing radiation. The presence of an accumulation of urine in the pyelo-calyceal cavities and the ureter, with varying degrees of dilatation, is a crucial need for a correct representation of the urinary excretory tract in this approach. The contrastographic impact is thus independent of renal function. This form of study is essential in patients who are allergic to contrast medium, in the investigation of living donor kidney transplantation, in uretero-hydronephrosis (particularly in pediatric patients and pregnant women), and as an alternative to uro-CT [42].
In conclusion, MRI can be used to research urinary stones because of its radiation-free nature and multiplanar imaging capabilities. It can help in stone detection and localization, as well as providing information on associated abnormalities. However, determining stone composition usually necessitates the use of other procedures, and MRI may not be as sensitive in detecting small or low-density stones.
It is important to have a clear pattern of action, a flow-chart, when there is a patient with acute flank pain to get an accurate diagnosis and a quick treatment (Fig. 5).
This image is a single energy non-contrast abdominal CT showing a stone formation in the right renal pelvis. By putting the ROI on the lithiasis formation we measure its density which comes out to be 135 HU, in this case: the calculations of uric acid have values of densities less than 500 HU. It is easier to see this differential using a CTDUAL ENERGY scan
Dual-Energy CT
Dual-Energy CT (DECT) is a novel technology for the study of urolithiasis that generates a variety of imaging datasets by simultaneously operating two X-ray tubes at two different kilovoltage levels. Its fundamental elements are the same as in traditional X-ray imaging: the X-ray tube’s power, the Compton and Photoelectric effect, and the material’s properties [43].
The DECT approach assumes that materials react differently to different X-ray photon energy. As a result, with the two different energies, each individual material received its suitable energy-dependent attenuation profiles.
The attenuation of photons as they pass through and interact with tissue is the basis for tissue distinction on CT and DECT. The attenuation, measured in Hounsfield Units (HU), is determined by the material’s characteristics, specifically its atomic number (Z) and electron density. Instead, it is inversely proportional to the radiant energy created by the tube (kVp): it is higher when low-energy photons are employed against high-energy photons [44•].
The interaction between photons and biological tissues depends on the Photoelectric and Compton effects at the energy levels employed in medical imaging. The Photoelectric effect is energy dependent and connected to atomic number (Z), but the Compton effect is energy independent and related to electron density (mass).
To summarize, DECT works on the premise of changes in photon absorption at different photon energy, which varies with material composition. As a result, each substance has its own energy. Soft tissues (liver, kidneys, pancreas, muscle, and fat) are made up of materials with similar Z values and exhibit no discernible difference in attenuation. Water, for example, has a straight line, whereas fat decreases attenuation values as we approach the lowest E (40–45 kEv).
The DECT acquisition methodology, as mentioned, is the same as the CTU acquisition protocol, which is a multi-phase acquisition. The initial step is an unenhanced scan of the abdomen and pelvis [45].
The patients are then administered furosemide intravenously to improve the delineation of the pelvicalyceal system and ureters and to generate more uniform opacification of the urine bladder.
The following are post-contrast phases: a corticomedullary phase obtained 30–40 s after the injection; a nephrographic phase obtained 90–110 s after contrast administration; and a pyelographic phase, a late excretory phase obtained 8–12 min after contrast medium was administered intravenously.
The unenhanced phase is used to detect stones, calcifications, hemorrhages, and clots, as well as to calculate the attenuation coefficients of urothelial masses; the corticomedullary phase is used to evaluate suspected vascular abnormalities or arterial enhancement; and the nephrographic phase is used to improve detection and characterization of renal lesions; a pyelographic phase is effective for assessing urothelial abnormalities such as distension and opacification of the collecting systems, ureters, and bladder [46].
DECT involves acquiring pictures with low-energy and high-energy X-rays, typically in the 80–140 kVp and 140–200 kVp ranges, respectively. Different materials have distinct spectral properties that allow for discrimination based on atomic composition. DECT can aid in the identification of stone types by evaluating their attenuation properties at various energy levels. The absorption patterns of various materials reveal information about the stone’s composition.
DECT produces color-coded images that emphasize the presence of various components in urinary calculi. Calcium oxalate stones, for example, may appear blue, whereas uric acid stones may appear red [47].
So, DECT is useful to determine the composition of the calculation [48]. In fact, it:
-
Is more effective than single energy CT.
-
Creates a better separation of stones from iodine.
-
Does better measures stone composition: better differentiation of urate stones from others (even at low doses).
In general, in clinical practice, CT is used to differentiate the calculations of uric acid from the calculations not of uric acid and it has been seen that the calculations of uric acid have values of densities less than 500 HU (Fig. 6).
In these dual-energy images (in a and b), there is the Color-Maps with the subtraction of hydroxyapatite: in fact, the compact bone (such as the spine and vertebrae) is colored in blue, in red there is acid uric. The proof of this is that: in the third image (c), there is instead the subtraction of uric acid and all that remain and is colored in red is hydroxyapatites (Color figure online)
DECT makes possible to separate the various components of the stone according to a color-code: all in relation to the hydroxyapatite, which is renowned for constituting compact bone (Figs. 7, 8 and 9).
Another very interesting application of CT (a simple CT scan without contrast medium) in general is the: maps of the atomic number (Fig. 10).
This is a flow chart for the imaging-indications to use in all patients with acute stone disease [49••]
In this way we could differentiate the elements that compose the calculation [49••].
Conclusions
Renal stone disease is a condition which creates acute discomfort and severe pain in the patient. Ultrasonography should always be used as a first approach, except in case of complications; instead, CT remains the gold standard in the diagnosis, and the indication of patient prognosis and treatment.
MRI has no indication, except for complications.
DECT, on the other hand, remains the most complete diagnostic choice for characterizing the anatomy, the cause, the extent of the stone and above all its composition, to choose the best therapy to treat the acute and to prevent relapses in the exposed patient.
Data Availability
We give our availability for data of this article.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•Pourvaziri A, Parakh A, Cao J, Locascio J, Eisner B, Sahani D, Kambadakone A. Comparison of four dual-energy CT scanner technologies for determining renal stone composition: a phantom approach. Radiology. 2022;304(3):580–9. https://doi.org/10.1148/radiol.210822. This reference compares four different DECT technologies to determine renal stone composition
Alahmadi AE, Aljuhani FM, Alshoabi SA, Aloufi KM, Alsharif WM, Alamri AM. The gap between ultrasonography and computed tomography in measuring the size of urinary calculi. J Family Med Primary Care. 2020;9(9):4925–8. https://doi.org/10.4103/jfmpc.jfmpc_742_20.
Chamanza R, Naylor SW, Carreira V, Amuzie C, Ma JY, Bradley AE, Blankenship B, McDorman K, Louden C. Normal anatomy, histology, and spontaneous pathology of the kidney, and selected renal biomarker reference ranges in the cynomolgus monkey. Toxicol Pathol. 2019;47(5):612–33. https://doi.org/10.1177/0192623319859263.
Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–9. https://doi.org/10.1159/000445450.
de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handbook Clin Neurol. 2015;130:61–108. https://doi.org/10.1016/B978-0-444-63247-0.00005-5.
Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P, Amundsen CL, Chai T, Close C, DiSanto M, Fraser MO, Kielb SJ, Kuchel G, Mueller ER, Palmer MH, Parker-Autry C, Wolfe AJ, Damaser MS. Sex differences in lower urinary tract biology and physiology. BMC, Biol Sex Differences. 2018;9:45. https://doi.org/10.1186/s13293-018-0204-8.
Siener R. Nutrition and kidney stone disease. Nutrients. 2021;13(6):1917. https://doi.org/10.3390/nu13061917.
Parks JH, Coward M, Coe FL. Correspondence between stone composition and urine supersaturation in nephrolithiasis. Kidney Int. 1997;51(3):894–900. https://doi.org/10.1038/ki.1997.126.
Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34:323–34. https://doi.org/10.1016/j.ucl.2007.04.009.
Laine C, Williams S, Goldfarb DS. In the clinic: nephrolithiasis. Ann Intern Med. 2009;151:ITC2. https://doi.org/10.7326/0003-4819-151-3-200908040-01002.
Corbo J, Wang J. Kidney and ureteral stones. Emerg Med Clin North Am. 2019;37(4):637–48. https://doi.org/10.1016/j.emc.2019.07.004.
Conrad S, Busch R, Huland H. Complicated urinary tract infections. Eur Urol. 1991;19(suppl 1):16–22. https://doi.org/10.1159/000473671.
Jha P, Bentley B, Behr S, Yee J, Zagoria R. Imaging of flank pain: readdressing state-of-the-art. Emerg Radiol. 2017;24:81–6. https://doi.org/10.1007/s10140-016-1443-9.
Viljoen A, Chaudhry R, Bycroft J. Renal stones. Ann Clin Biochem. 2019;56(1):15–27. https://doi.org/10.1177/0004563218781672.
Miller NL, Evan AP, Lingeman JE. Pathogenesis of renal calculi. Urol Clin North Am. 2007;34(3):295–313. https://doi.org/10.1016/j.ucl.2007.05.007.
Ferraro PM, Curhan GC, D’Addessi A, Gambaro G. Risk of recurrence of idiopathic calcium kidney stones: analysis of data from the literature. J Nephrol. 2017;30(2):227–33. https://doi.org/10.1007/s40620-016-0283-8.
Griffith DP. Struvite stones. Kidney Int. 1978;13(5):372–82. https://doi.org/10.1038/ki.1978.55.
Liebman SE, Taylor JG, Bushinsky DA. Idiopathic hypercalciuria. Curr Rheumatol Rep. 2006;8:70–5. https://doi.org/10.1007/s11926-006-0029-z.
Fowler KA, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222(1):109–13. https://doi.org/10.1148/radiol.2221010453.
Ganesan V, De S, Greene D, Torricelli FC, Monga M. Accuracy of ultrasonography for renal stone detection and size determination: is it good enough for management decisions? BJU Int. 2017;119(3):464–9. https://doi.org/10.1111/bju.13605.
Taylor DZ, Smith GE, Wiener SV. Identification of clinically insignificant renal calculi on sonography. Urology. 2023. https://doi.org/10.1016/j.urology.2023.03.020.
AlSaiady M, Alqatie A, Almushayqih M. Twinkle artifact in renal ultrasound, is it a solid point for the diagnosis of renal stone in children? J Ultrason. 2021;21(87):e282–5. https://doi.org/10.15557/JoU.2021.0048.
Tchelepi H, Ralls PW. Color comet-tail artifact: clinical applications. AJR Am J Roentgenol. 2009;192(1):11–8. https://doi.org/10.2214/AJR.07.3893.
Hassan W, Sharif I, Elkhalid S, Ellahibux K, Sultan S, Waqar A, Zohaib A, Yousuf F. Doppler-assessed ureteric jet frequency: a valuable predictor of ureteric obstruction. Cureus. 2021;13(9):e18290. https://doi.org/10.7759/cureus.18290.
Cox IH, Erickson SJ, Foley WD. Dewire DM Ureteric jets: evaluation of normal flow dynamics with color Doppler sonography. AJR Am J Roentgenol. 1992;158:1051–5. https://doi.org/10.2214/ajr.158.5.1566665.
Katz D, McGahan JP, Gerscovich EO, Troxel SA, Low RK. Correlation of ureteral stone measurements by CT and plain film radiography: utility of the KUB. J Endourol. 2003;17(10):847–50. https://doi.org/10.1089/089277903772036118.
Roth CS, Bowyer BA, Berquist TH. Utility of the plain abdominal radiograph for diagnosing ureteral calculi. Ann Emerg Med. 1985;14(4):311–5. https://doi.org/10.1016/s0196-0644(85)80094-9.
•Hsieh TY, Chen SL, Chang YR, Tyan YS, Chen TR. Effective dose for kidney-ureter-bladder plain radiography, intravenous urography, and abdominal computed tomography scan: A phantom study. Appl Radiat Isot. 2022;187:110339. https://doi.org/10.1016/j.apradiso.2022.110339. This reference demonstrates a study to compare the effective dose for plain radiograph, intravenous urography and CT, very important as regards exposure in subjects at risk, such as pregnant women or children.
Costello CH, Cook CK. Intravenous urography and imaging of the urinary tract. Hosp Med. 2004;65(7):426–30. https://doi.org/10.12968/hosp.2004.65.7.15479.
Shine S. Urinary calculus: IVU vs CT renal stone? A critically appraised topic. Abdom Imaging. 2008;33:41–3. https://doi.org/10.1007/s00261-007-9307-0.
Stacul F, Rossi A, Cova MA. CT urography: the end of IVU? Radiol Med. 2008;113(5):658–69. https://doi.org/10.1007/s11547-008-0281-6.
Kennish SJ, Wah TM, Irving HC. Unenhanced CT for the evaluation of acute ureteric colic: the essential pictorial guide. Postgrad Med J. 2010;86(1017):428–36. https://doi.org/10.1136/pgmj.2009.084954.
Smith D, Patel U. Ultrasonography vs. computed tomography for stone size. BJU Int. 2017;119(3):361–2. https://doi.org/10.1111/bju.13735.
Mostafavi MR, Ernst RD, Saltzman B. Accurate determination of chemical composition of urinary calculi by spiral computerized tomography. J Urol. 1998. https://doi.org/10.1016/S0022-5347(01)63698-X.
Kawashima A, Sandler CM, Boridy IC, Takahashi N, Benson GS, Goldman SM. Unenhanced helical CT of ureterolithiasis: value of the tissue rim sign. AJR Am J Roentgenol. 1997;168(4):997–1000. https://doi.org/10.2214/ajr.168.4.9124157.
Carius BM, Long B. Is this your stone? Distinguishing phleboliths and nephroliths on imaging in the emergency department setting. J Emerg Med. 2022;62(3):316–23. https://doi.org/10.1016/j.jemermed.2021.10.034.
Akbar SA, Mortele KJ, Baeyens K, Kekelidze M, Silverman SG. Multidetector CT urography: techniques, clinical applications, and pitfalls. Semin Ultrasound CT MR. 2004;25(1):41–54. https://doi.org/10.1053/j.sult.2003.11.002.
Kemper J, Regier M, Stork A, Adam G, Nolte-Ernsting C. Improved visualization of the urinary tract in multidetector CT urography (MDCTU): analysis of individual acquisition delay and opacification using furosemide and low-dose test images. J Comput Assist Tomogr. 2006;30(5):751–7. https://doi.org/10.1097/01.rct.0000224631.25198.ed.
Hiorns MP. Imaging of the urinary tract: the role of CT and MRI. Pediatr Nephrol. 2011;26(1):59–68. https://doi.org/10.1007/s00467-010-1645-4.
Kalb B, Sharma P, Salman K, Ogan K, Pattaras JG, Martin DR. Acute abdominal pain: is there a potential role for MRI in the setting of the emergency department in a patient with renal calculi? J Magn Reson Imaging. 2010;32(5):1012–23. https://doi.org/10.1002/jmri.22337.
Otero HJ, Elsingergy MM, Back SJ. Magnetic resonance urography: a practical approach to preparation, protocol and interpretation. Pediatr Radiol. 2022. https://doi.org/10.1007/s00247-022-05511-7.
Leyendecker JR, Barnes CE, Zagoria RJ. MR urography: techniques and clinical applications. Radiographics. 2008;28(1):23–46. https://doi.org/10.1148/rg.281075077.
Agostini A, Borgheresi A, Mari A, Floridi C, Bruno F, Carotti M, Schicchi N, Barile A, Maggi S, Giovagnoni A. Dual-energy CT: theoretical principles and clinical applications. Radiol Med. 2019;124(12):1281–95. https://doi.org/10.1007/s11547-019-01107-8.
•Borges AP, Antunes C, Curvo-Semedo L. Pros and cons of dual-energy ct systems: “one does not fit all”. Tomography. 2023;9(1):195–216. https://doi.org/10.3390/tomography9010017. This study reference compares the pros and cons of the various technologies that DECT uses to study calculations.
Mansouri M, Aran S, Singh A, Kambadakone AR, Sahani DV, Lev MH, Abujudeh HH. Dual-energy computed tomography characterization of urinary calculi: basic principles, applications and concerns. Curr Probl Diagn Radiol. 2015;44(6):496–500. https://doi.org/10.1067/j.cpradiol.2015.04.003.
Euler A, Wullschleger S, Sartoretti T, Müller D, Keller EX, Lavrek D, Donati O. Dual-energy CT kidney stone characterization-can diagnostic accuracy be achieved at low radiation dose? Eur Radiol. 2023. https://doi.org/10.1007/s00330-023-09569-1.
Kapanadze LB, Rudenko VI, Serova NS, Rapoport LM, Aleksandrova KA, Novikov AA. Dual-energy computed tomography in the diagnostics of urolithiasis. Urologiia. 2019;5:31–6. https://doi.org/10.18565/urology.2019.5.31-36.
••Nourian A, Ghiraldi E, Friedlander JI. Dual-energy CT for urinary stone evaluation. Curr Urol Rep. 2020;22(1):1. https://doi.org/10.1007/s11934-020-01019-5. This reference demonstrates a study evaluating the possible use of DECT in the study of kidney stones
••Skolarikos A, Jung H, Neisius A, Petřík A, Somani B, Tailly T, Gambaro G, Davis NF, Geraghty R, Lombardo R, Tzelves L, Shepherd R. EAU guidelines on urolithiasis. European Association of Urology. 2023. https://uroweb.org/urolithiasis/chapter/guidelines. This reference updates the guidelines, updated every year by the European Society of Urology, on the diagnosis, management and treatment of urolithiasis.
Acknowledgements
We thank Dr. Luca Saba for reviewing the manuscript.
Funding
Open access funding provided by Università di Foggia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors contributed to the article’s conception and design. All authors read and approved the final manuscript. The authors have no financial or competing interests to disclose.
Human/Animal Studies Informed Consent Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to Publish
All the figures and tables using in this article are original.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montatore, M., Muscatella, G., Eusebi, L. et al. Current Status on New Technique and Protocol in Urinary Stone Disease. Curr Radiol Rep 11, 161–176 (2023). https://doi.org/10.1007/s40134-023-00420-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40134-023-00420-5