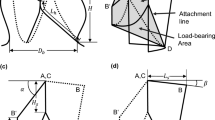
Abstract
Atrial septal defects are the most common congenital cardiac defects. The natural history of an uncorrected atrial septal defect causes a shortened life expectancy due to right ventricular volume overload and associated congestive heart failure, atrial arrhythmias, and/or pulmonary vascular disease. Surgical closure of the atrial septal defect is a procedure with a long-standing history, and the maturing field of percutaneous closure of atrial septal defects by device implantation has established itself to be a feasible, minimally invasive, and safe procedure. Inherent limitations in device designs have resulted in rare, but serious complications, through subsequent changes in the technical aspects of transcatheter atrial septal defect closure that have minimized the number of patients with an adverse event. Recent Food and Drug Administration re-evaluation of the safety of atrial septal defect closure by device has brought to light some of the acute and long-term issues related to device occlusion. This paper summarizes the history of atrial septal defects, surgical and transcatheter device closure, and the most current outcomes of percutaneous atrial septal defect device occlusion in the pediatric population.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Samanek M, Vorísková M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15-year survival: a prospective Bohemia survival study. Pediatr Cardiol. 1999;20:411–7.
Veldtman GR, Freedom RM, Benson LN. Atrial septal defect. In: Yoo S-J, Freedom RM, Mikailian H, Williams WG, editors. The natural and modified history of congenital heart disease. New York: Blackwell Publishing; 2004. p. 31–43.
Ferreira Martins JD, Anderson R. The anatomy of interatrial communications—what does the interventionist need to know? Cardiol Young. 2000;10:464–73.
Borow KM, Karp R. Atrial septal defect. Lessons from the past, directions for the future. N Engl J Med. 1990;323:1698–700.
Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Zahn EM. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the american heart association. Circulation. 2011;2011(123):2607–52.
Campbell M. Natural history of atrial septal defect. Br Heart J. 1970;32:820–6.
Garg G, Tyagi H, Radha AS. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein to superior vena cava—an innovative technique. Catheter Cardiovasc Interv. 2014;84:473–7.
Murray G. Closure of defects in the cardiac septa. Ann Surg. 1948;128:843–50.
Kirklin JW, Barratt-Boyes BG. Cardiac surgery. New York: Churchill Livingstone; 1993. p. 609–44.
Mills NL, King TD. Nonoperative closure of left-to-right shunts. J Thorac Cardiovasc Surg. 1976;72:371–8.
Mills NL, King TD. Late follow-up of nonoperative closure of secundum atrial septal defects using the King-Mills double-umbrella device. Am J Cardiol. 2003;92:353–5.
Prieto LR, Foreman CK, Cheatham JP, Latson LA. Intermediate-term outcome of transcatheter secundum atrial septal defect closure using the Bard Clamshell Septal Umbrella. Am J Cardiol. 1996;78:1310–2.
Carminati M, Giusti S, Hausdorf G, Qureshi S, Tynan M, Witsenburg M, DeGeeter B. A European multicentric experience using the Cardio SEal and Starflex double umbrella devices to close interatrial communications holes with the oval fossa. Cardiol Young. 2000;10:519–26.
Rao PS, Sideris EB. Centering-on-demand buttoned device: its role in transcatheter occlusion of atrial septal defects. J Interv Cardiol. 2001;14:81–9.
Morgan G, Lee KJ, Chaturvedi R, Benson L. A biodegradable device (BioSTAR) for atrial septal defect closure in children. Catheter Cardiovasc Interv. 2010;76:241–5.
•• Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–44. This publication altered the outcomes of patients with an ASD by expanding the procedural options for closure. This shifted the landscape in the U.S.A. for defect closure.
• Jones TK, Latson LA, Zahn E, Fleishman CE, Jacobson J, Vincent R, Kanter K. Results of the U.S. multicenter pivotal study of the HELEX septal occluder for percutaneous closure of secundum atrial septal defects. J Am Coll Cardiol. 2007;49:2215–21. This publication expanded the options for interventional cardiologists in the U.S.A. and has offered an additional device for safe closure.
MacDonald ST, Daniel MJ, Ormerod OJ. Initial use of the new GORE® septal occluder in patent foramen ovale closure: implantation and preliminary results. Catheter Cardiovasc Interv. 2013;81:660–5.
• Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502. The reporting of device erosion and associated risk factors was an integral step in the process of defining septal rims and adjusting technical aspects of implantation to improve outcomes.
Qureshi AM, Mumtaz MA, Latson LA. Partial prolapse of a HELEX device associated with early frame fracture and mitral valve perforation. Catheter Cardiovasc Interv. 2009;74:777–82.
Levi DS, Moore JW. Embolization and retrieval of the Amplatzer Septal occluder. Catheter Cardiovasc Interv. 2004;61:543–7.
Rigatelli G, Dell’Avvocata F, Cardaioli P, Giordan M, Dung HT, Nghia NT, Nanjiundappa A. Safety and long-term outcome of modified intracardiac echocardiography-assisted “no-balloon” sizing technique for transcatheter closure of ostium secundum atrial septal defect. J Interv Cardiol. 2012;25:628–34.
Tzifa A, Gordon J, Tibby SM, Rosenthal E, Qureshi SA. Transcatheter atrial septal defect closure guided by colour flow Doppler. Int J Cardiol. 2011;149:299–303.
Roos-Hesselink JW, Meijboom FJ, Spitaels SEC, Van Domburg R, van Rijen EHM, Utens EMWJ, Simoons ML. Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age: a prospective follow-up study of 21-33 years. Eur Heart J. 2003;24:190–7.
Suchon E, Pieculewicz M, Tracz W, Przewlocki T, Sadowski J, Podolec P. Transcatheter closure as an alternative and equivalent method to the surgical treatment of atrial septal defect in adults: comparison of early and late results. Med Sci Monit. 2009;15:CR612–7.
Kaya MG, Baykan A, Dogan A, Inanc T, Gunebakmaz O, Dogdu O, Narin N. Intermediate-term effects of transcatheter secundum atrial septal defect closure on cardiac remodeling in children and adults. Pediatr Cardiol. 2010;31(4):474–82.
Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM. Long-term follow up of secundum atrial septal defect closure with the Amplatzer septal occluder. Congenit Heart Dis. 2010;5:32–7.
Walters DL, Boga T, Burstow D, Scalia G, Hourigan LA, Aroney CN. Percutaneous ASD closure in a large Australian series: short- and long-term outcomes. Heart Lung Circ. 2012;21:572–5.
DiBardino DJ, McElhinney DB, Kaza AK, Mayer JE. Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg. 2009;137:1334–41.
Visconti KJ, Bichell DP, Jonas RA, Newburger JW, Bellinger DC. Developmental outcome after surgical versus interventional closure of secundum atrial septal defect in children. Circulation. 1999;100(suppl 2):II–145.
Bartakian S, Fagan TE, Schaffer MS, Darst JR. Device closure of secundum atrial septal defects in children <15 kg: complication Rates and Indications for Referral. J Am Coll Cardiol. 2012;5:1178–84.
Petit CJ, Justino H, Pignatelli RH, Crystal MA, Payne WA, Ing FF. Percutaneous atrial septal defect closure in infants and toddlers: predictors of success. Pediatr Cardiol. 2013;34:220–5.
Du ZD, Koenig P, Cao QL, Waight D, Heitschmidt M, Hijazi ZM. Comparison of transcatheter closure of secundum defects using the Amplatzer septal occluder associated with deficient versus sufficient rims. Am J Cardiol. 2002;90:865–9.
Moore J, Hegde S, El-Said H, Beekman R, Bergersen L, Martin G. Transcatheter device closure of atrial septal defects: a safety review. JACC. 2013;6:433–42.
• Ami Z, Echocardiographic predictors of cardiac erosion after Amplatzer Septal occluder placement. Catheter Cardiovasc Interv. 2014;83:84–92. This paper outlines new potential risks for device erosion, anatomic and device variants to assess and recommendations on avoidance of erosion.
Taggart NW, Dearani JA, Hagler DJ. Late erosion of an Amplatzer septal occluder device 6 years after placement. J Thorac Cardiovasc Surg. 2011;142:221–2.
Johnson JN, Marquardt ML, Ackerman MJ, Asirvatham SJ, Reeder GS, Cabalka AK, Hagler DJ. Electrocardiographic changes and arrhythmias following percutaneous atrial septal defect and patent foramen ovale device closure. Catheter Cardiovasc Interv. 2011;78(2):254–61.
Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patent foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–9.
Freixa X, Ibrahim R, Chan J, Garceau P, Dore A, Marcotte F, Asgar AW. Initial clinical experience with the Gore septal occluder for the treatment of atrial septal defects and patent foramen ovale. EuroIntervention. 2013;9:629–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Matthew A. Crystal and Julie A. Vincent declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardiology.
Rights and permissions
About this article
Cite this article
Crystal, M.A., Vincent, J.A. Atrial Septal Defect Device Closure in the Pediatric Population: A Current Review. Curr Pediatr Rep 3, 237–244 (2015). https://doi.org/10.1007/s40124-015-0086-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-015-0086-8