Abstract
Introduction
To evaluate the antimicrobial efficacy of an ophthalmic formulation containing hexamidine diisethionate (HD) 0.05%, polyhexamethylene biguanide (PHMB) 0.0001%, and edetate disodium (EDTA) 0.01% (Keratosept®, Bruschettini, Genova, Italy) on the microbial flora of a healthy ocular surface.
Methods
Patients were enrolled consecutively. Each patient applied two drops of Keratosept® in the eye scheduled for cataract surgery (study eye) three times daily in the 2 days prior to surgery and one time in the morning of surgery. The contralateral eyes were considered as control (control eye). Bilateral conjunctival swabs were collected before the first administration (T0) and the morning of surgery (T1). The swabs were processed within 3 h from sampling for the automated detection of the presence of replicating microorganisms (colony-forming units, CFU/mL) and the provision of real-time growth curves.
Results
Conjunctival swabs of 32 patients (n = 128) were examined. Six patients were excluded from the efficacy analysis because of microbial load < 50 CFU/mL at T0 in the study eye. No difference between study and control eyes was observed at T0 (p = 0.40). Compared with T0, 20 (76.9%) study eyes and 10 (38.5%) control eyes showed a ≥ 1 log reduction of the microbial load at T1, with a significant difference between groups (p = 0.005). Keratosept® showed good tolerability, and no adverse events or eye discomfort were recorded.
Conclusions
This study showed that the low-dose combination of antiseptic agents in the Keratosept® ophthalmic solution effectively reduces the bacterial load of healthy flora on the ocular surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Topical formulations containing antiseptics could address the growing issue of antibiotic resistance and represent a viable alternative to preoperative antibiotic prophylaxis. |
The antimicrobial activity of Keratosept® ophthalmic solution has already been assessed in vitro on bacterial and fungal isolates, showing promising antimicrobial activity. |
We evaluated the antimicrobial efficacy of an ophthalmic formulation containing hexamidine diisethionate (HD) 0.05%, polyhexamethylene biguanide (PHMB) 0.0001%, and edetate disodium (EDTA) 0.01% on the microbial flora of healthy ocular surface. |
This low-dose combination of antiseptic agents in ophthalmic solution effectively reduces the bacterial load of healthy flora on the ocular surface. |
Introduction
Millions of cataract surgeries are performed globally every year, with demand continuing to grow, as the World Health Organization (WHO) estimates that by 2030, one in six people will be 60 years of age or older, and this number will double by 2050 [1].
Despite its incidence dropping to 0.014–0.08% with routine use of intracameral cefuroxime, post-surgical endophthalmitis still represents a sight-threatening complication typically caused by microorganisms deriving from the patient’s ocular surface flora [2,3,4]. Moreover, the misuse of topical antibiotics strongly contributes to the spread of antibiotic-resistant ocular pathogens [5]. The ongoing Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) study, the largest prospective study on antimicrobial susceptibility of common ocular pathogens to date, has shown an alarming rate of multidrug resistance (≥ 3 antibiotic classes), up to 30.2% for methicillin-resistant Staphylococcus aureus (MRSA) and 39% for coagulase-negative staphylococci (CoNS) [6].
Topical formulations containing antiseptics could help address the growing issue of antibiotic resistance and represent a viable alternative to preoperative antibiotic prophylaxis. Hexamidine diisethionate (HD), polyhexamethylene biguanide (PHMB), and edetate disodium (EDTA) have well-known antiseptic properties with a wide antimicrobial spectrum, including fungi and protozoa, and high biocompatibility. HD and PHMB are strong bases that interact with negatively charged phospholipids in the bacterial membrane [7, 8]. EDTA is a chelating agent that binds to Mg2+ and Ca2+ ions on the oligosaccharide chain of the lipopolysaccharide and disrupts the integrity of Gram-negative bacteria. Similarly, chelation of Fe+ and Ca2+ explains its inhibitory effect on fungal growth [9].
The antimicrobial activity of Keratosept® ophthalmic solution has already been assessed in vitro on bacterial and fungal isolates, showing promising antimicrobial activity mainly against Streptococcus, Staphylococcus, and Candida spp. [10, 11].
In this study, we evaluated the antimicrobial efficacy and tolerability of Keratosept® on the resident ocular surface microbial flora of healthy patients scheduled for cataract surgery.
Methods
Study Design
This is a prospective, single-center clinical study. The study protocol was approved by the Ethics Committee of Venice and San Camillo Research Institute (ref. no. 1433; executive determination no. 210A/CESC, February 22, 2022). Written informed consent was obtained before the first administration of the ophthalmic solution, and the study was conducted following the tenets of the Declaration of Helsinki.
Study Population and Protocol
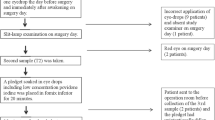
Thirty-two consecutive patients (64 eyes) between 40 and 90 years of age scheduled for cataract surgery in one eye were enrolled in the study between May 2022 and July 2022 at the Hospital of Chioggia (Chioggia, Italy). All patients received cataract surgery in the second eye within 1 month from the first eye, given the massive impact of COVID-19 on the healthcare system. Patients with signs of ocular inflammation/infection, ongoing topical treatments, autoimmune diseases, use of antibiotics during the last 3 months, hypersensitivity to one or more components of the study treatment, and/or ongoing participation in other clinical trials were excluded.
Each patient applied two drops of Keratosept® in the eye scheduled for cataract surgery (study eye) three times daily in the 2 days prior to surgery and two drops the morning of surgery. The contralateral eye was considered as control (control eye).
Bilateral conjunctival swabs (FLOQSwabs®, COPAN Group, Brescia, Italy) were collected at the baseline visit 2 days before cataract surgery (T0) and the morning of cataract surgery 30 min prior to entering the operating room (T1). The same operator (RLM) collected the conjunctival samples by rotational movements of the swab three times in the inferior fornix after retracting the lid margin and avoiding touching it to prevent any potential contamination. No topical anesthesia was applied. Conjunctival swabs were sent to the laboratory of Fondazione Banca degli Occhi (Venice, Italy) in a modified liquid Amies medium (ESwab®, COPAN Group, Brescia, Italy) within 3 h from the collection.
A complete ocular examination, including visual acuity, fluorescein staining, conjunctival hyperemia, and intraocular pressure, was performed at T0 and T1 (as specifically established by the study protocol) and after cataract surgery at 1 day and 30 days (according to the current hospital procedures).
The same operator (MT) performed cataract surgeries by phacoemulsification and intraocular lens implantation. Before each surgical procedure, the eyelids and periocular skin area were disinfected with povidone-iodine (PVI) 10%, and an ophthalmic solution containing PVI 5% was instilled in the eye. Adverse events were recorded.
Microbiological Analysis
After the incubation of the sample for 24 h at 30 °C, 0.5 mL of medium was inoculated onto a specific culture broth and incubated at 37 °C for a further 24 h in the HB&L system (Alifax, Polverara, Italy) to obtain the microbial culture and monitor the bacterial replication phase. Based on light-scattering technology, the HB&L reveals the presence of replicating microorganisms and provides real-time growth curves and quantitative microbial count results (microbial load) in terms of colony-forming units (CFU)/mL.
To check for the presence of residual antimicrobial agents in the samples and avoid false-negative results, we measured the residual antimicrobial activity (RAA) by incubation of 0.5 mL of medium in a culture containing S. epidermidis as a control run at the same time as the patients’ samples.
A microbial load reduction of ≥ 1 log at T1 was considered a favorable outcome and indicative of a significant effect of the ophthalmic solution on the resident flora.
Statistical Analysis
Categorical variables (sex, adverse events, ocular surface evaluation) are given as absolute numbers and percentages. Continuous variables (microbial load, age) are given as mean and standard deviation (SD) in the case of normal distribution; otherwise, median and 95% CI.
Conjunctival swabs with less than 50 CFU/mL were considered not useful for the evaluation of the efficacy of the ophthalmic solution.
The Student t-test and Wilcoxon signed-rank test were used for comparison of bacterial load at T0 and T1 in the case of normal and non-normal distribution, respectively. The McNemar and Bowker tests were used to compare two or more categorical variables, respectively. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 32 patients—15 female and 17 male, aged 75.6 (± 9) years—and 128 conjunctival swabs were examined. Six patients were excluded from the efficacy analysis because the conjunctival swabs at T0 from the study eyes yielded less than 50 CFU/mL.
At T0, study and control eyes showed no differences in the microbial load (p = 0.40), revealing that study and control eyes were not different at baseline with respect to the microbial load.
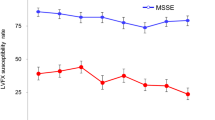
Although at T1 the microbial load was not statistically different from that at T0 in either group (p = 0.17), a greater overall decrease in microbial load was found in the study versus control eyes (Table 1 and Fig. 1). However, when considering the eyes with a bacterial load reduction ≥ 1 log at T1, 20 (76.9%) study eyes versus 10 (38.5%) control eyes reached the goal, with a significant difference between the two groups (p = 0.005) (Fig. 2).
Distribution of CFU in study and control eyes. Scores showed reduction from T0 to T1 in all groups (n = 26). The boxes represent the 25th and 75th percentiles; whiskers are lines extending from each end of the box to the minimum or maximum or the lowest datum within 1.5-fold IQR of the lower quartile or the highest datum within 1.5-fold IQR of the upper quartile. The median value is the line that bisects the boxes, and the circles are the outlier values. T0 time point 0, before the first administration; T1 time point 1, morning of surgery; CFU colony-forming unit; IQR interquartile range
The RAA test excluded false-negative results. None of the patients showed signs of conjunctival or corneal toxicity or inflammation, nor did any report ocular discomfort related to the use of Keratosept®. Normal intraocular pressure (IOP) values were maintained. No cases of postoperative endophthalmitis or other ocular surface infectious disorders were recorded up to 1-month follow-up and thereafter.
Discussion
The spread of antibiotic-resistant microorganisms has dramatically increased the need for an appropriate prophylaxis for infectious complications. Ophthalmologists are particularly exposed to this matter since some surgical procedures, such as cataract surgery and intravitreal anti-vascular endothelial growth factor (VEGF) injection, are by far the most commonly performed worldwide. Antibiotics have long been a cornerstone of prevention even though there is no evidence of the benefits of topical antibiotics over chlorhexidine, PVI prophylaxis, or intracameral cefuroxime alone [12, 13]. The evidence of both reduced susceptibility of MRSA and other staphylococci to chlorhexidine and the corneal cytotoxicity of high-concentration PVI [14, 15] is prompting the search for new formulations and molecules with a broad spectrum of antimicrobial activity and high ocular tolerability [16,17,18]. To this end, a combination of different antiseptics, such as HD, PHMB, and EDTA in Keratosept®, could help in preventing resistance to a single antimicrobial agent.
Although several antiseptic agents have proven inhibitory power over microbial isolates when assessed in vitro, application in the patient is much different, since blinking, dilution of the ophthalmic solution onto the ocular surface, compliance, and accurate administration are some of the variables to consider. In this regard, a recently published study found comparable in vivo efficacy profiles of Keratosept® and PVI 0.6% in 50 consecutive patients receiving intravitreal anti-VEGF agents, with a better tolerability profile for Keratosept than for PVI [19].
This is the first in vivo clinical assessment of the antimicrobial efficacy and ocular tolerability of an ophthalmic solution combining three different low-dose antiseptics in patients undergoing cataract surgery. The preliminary results showed that a 2-day course of Keratosept® affected the resident healthy microbial flora in patients scheduled for cataract surgery, leading to a decrease in microbial load after short-term application.
We found no overall statistically significant decrease in microbial load from T0 to T1 in the study versus control eyes, a result in line with that obtained from other authors who applied the technique based on HB&L to detect positive swabs [20]. In fact, test results expressed in terms of CFU showed high variability, possibly as a consequence of induction of reflex tearing when touching the conjunctiva, causing sample dilution.
When focusing on cases with favorable outcomes (reduction of microbial load ≥ 1 log at T1 compared to T0), a significantly different proportion between study eyes (76.9%) and control eyes (38.5%) can only be attributed to the use of Keratosept®. The favorable outcome in control eyes could be explained as a result of microbial load dilution by reflex tearing, since the conjunctival swab of the control eye was always obtained after that of the treated eye. Six patients (19%) were excluded from the efficacy analysis, in line with a previous study [18].
The use of Keratosept® was confirmed safe, and no signs of toxicity or subjective discomfort were reported. With regard to the occurrence of endophthalmitis or other postoperative infections, given their extremely low prevalence, the number of patients included in the study was inadequate to retrieve any possible events.
Study Limitations
The authors are aware of the limitations of the results from this study, mainly the small sample size, the lack of a comparator topical antiseptic, the blinding condition, and the absence of specific tools (e.g., conjunctival hyperemia scale, Ocular Surface Disease Index [OSDI], 10-point visual analog scale) to assess the tolerability of the ophthalmic solution.
Conclusions
Our study represents the first clinical assessment of the antimicrobial activity and ocular tolerability of an ophthalmic solution combining three low-dose antiseptics in patients undergoing cataract surgery. The results support the antimicrobial activity of the Keratosept® ophthalmic solution on the resident normal ocular surface flora. Further studies could clarify whether different treatment schemes in terms of dosage and duration may be associated with greater effectiveness.
Data Availability
The data used or analyzed during the current study are available from the corresponding author upon request.
References
World Health Organization. Ageing and health. Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88.
Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32:407–10.
Althiabi S, Aljbreen AJ, Alshutily A, Althwiny FA. Postoperative endophthalmitis after cataract surgery: an update. Cureus. 2022;14: e22003.
World Health Organization. Antibiotic resistance. Available at: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.
Asbell PA, DeCory HH. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. PLoS ONE. 2018;13:1–17.
Hübner NO, Kramer A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 2010;23:17–27.
Lee J-E, Oum BS, Choi HY, Yu HS, Lee JS. Cysticidal effect on acanthamoeba and toxicity on human keratocytes by polyhexamethylene biguanide and chlorhexidine. Cornea. 2007;26:736–41.
Percival SL, Salisbury AM. The efficacy of tetrasodium EDTA on biofilms. Adv Exp Med Biol. 2018;1057:101–10.
Pinna A, Donadu MG, Usai D, Dore S, Boscia F, Zanetti S. In vitro antimicrobial activity of a new ophthalmic solution containing hexamidine diisethionate 0.05% (Keratosept). Cornea. 2020;39:1415–8.
Mencucci R, Favuzza E, Bottino P, Mazzantini C, Zanotto E, Pellegrini-Giampietro DE, Landucci E. A new ophthalmic formulation containing antiseptics and dexpanthenol: In vitro antimicrobial activity and effects on corneal and conjunctival epithelial cells. Exp Eye Res. 2020;201: 108269.
Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15–21.
Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2002;28:977–81.
Horner C, Mawer D, Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother. 2012;67:2547–59.
Shibata Y, Tanaka Y, Tomita T, et al. Evaluation of corneal damage caused by iodine preparations using human corneal epithelial cells. Jpn J Ophthalmol. 2014;58:522–7.
Spadea L, Zanotto E, Cavallo R, Campagna G, Giannico MI, Costagliola C. Effectiveness of liposomal ozonized oil in reducing ocular microbial flora in patients undergoing cataract surgery. J Cataract Refract Surg. 2021;47:1548–55.
Reibaldi M, Avitabile T, Bandello F, et al. The effectiveness of 0.6% povidone iodine eye drops in reducing the conjunctival bacterial load and needle contamination in patients undergoing anti-VEGF intravitreal injection: A prospective, randomized study. J Clin Med. 2019;8:1031.
Musumeci R, Bandello F, Martinelli M, Calaresu E, Cocuzza CE. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur J Ophthalmol. 2019;29:673–7.
Avogaro F, Florido A, Calandri A, Malvasi M, Vingolo EM. Intravitreal injections primary prevention: a case-control study. Eur Rev Med Pharmacol Sci. 2023;27:3664–9.
Giannaccare G, Comis S, Jannuzzi V, et al. Effect of liposomal-lactoferrin-based eye drops on the conjunctival microflora of patients undergoing cataract surgery. Ophthalmol Ther. 2023;12:1315–26.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Editorial assistance was provided by Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by Bruschettini.
Funding
This research received no external funding. The Rapid Service Fee was funded by Bruschettini.
Author information
Authors and Affiliations
Contributions
Adriano Fasolo, Diego Ponzin and Marco Tavolato designed the research; Davide Camposampiero, Marco Tavolato and Rocco Luigi Modugno performed the research; Rocco Luigi Modugno, Adriano Fasolo, Davide Camposampiero, Diego Ponzin and Marco Tavolato collected and interpreted data; Adriano Fasolo and Davide Camposampiero analyzed the data; Adriano Fasolo, Rocco Luigi Modugno and Marco Tavolato wrote the manuscript. Rocco Luigi Modugno, Adriano Fasolo, Davide Camposampiero, Diego Ponzin and Marco Tavolato edited the manuscript; Rocco Luigi Modugno, Adriano Fasolo, Davide Camposampiero, Diego Ponzin and Marco Tavolato approved the paper submission.
Corresponding author
Ethics declarations
Conflict of Interest
Rocco Luigi Modugno, Adriano Fasolo, Davide Camposampiero, Diego Ponzin and Marco Tavolato have no conflicts of interest to disclose.
Ethical Approval
The study protocol was approved by the Ethics Committee of Venice and San Camillo Research Institute (ref. no. 1433; executive determination no. 210A/CESC, February 22, 2022). Written informed consent was obtained before the first administration of the ophthalmic solution, and the study was conducted following the tenets of the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Modugno, R.L., Fasolo, A., Camposampiero, D. et al. Efficacy and Safety of Preoperative Prophylaxis in Cataract Surgery with Combined Topical Antiseptics: A Microbiological Study. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-01000-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-01000-2