Abstract
Introduction
Highly myopic macular hole retinal detachment (MHRD) is often associated with a poor prognosis, and there is currently no optimal treatment. Platelet-rich fibrin (PRF), an autologous blood product, has been shown to promote tissue regeneration. This prospective, randomized, controlled study investigated the efficacy of conventional internal limiting membrane (ILM) peeling versus PRF membrane transplantation in highly myopic MHRD.
Methods
Eyes with highly myopic MHRD were randomly assigned to either a conventional ILM peeling group (IP group, n = 19) or a PRF membrane transplantation group (PMT group, n = 21). The study followed participants for a period of 6 months. The primary outcome measure was macular hole (MH) closure assessed using optical coherence tomography. Secondary outcomes included best-corrected visual acuity (BCVA), central retinal thickness (CRT), superficial vascular density (SVD), deep vascular density (DVD), rate of retinal reattachment, and any complications encountered.
Results
MH closure was achieved in a significantly greater proportion of eyes in the PMT group (21/21, 100.00%) compared to the IP group (15/19, 78.95%) (P = 0.042). Retinal reattachment was accomplished in all patients (100.00%) within both groups. Except for an insignificant difference in BCVA observed at 1 week post-surgery in the IP group, significant improvements in BCVA and CRT were documented in both groups across all other post-operative time points. Final BCVA (P = 0.040), CRT (P = 0.002), SVD (P = 0.002), and DVD (P = 0.013) were all significantly higher in the PMT group compared to the IP group. No serious complications were identified in either group.
Conclusions
This study demonstrated the superiority of PRF membrane transplantation compared to conventional ILM peeling in promoting MH closure and enhancing retinal vascular density in patients with highly myopic MHRD. Additionally, PRF membrane transplantation effectively restores retinal reattachment, improves visual function, and increases retinal thickness without introducing additional complications.
Trial Registration Number
www.clinicaltrials.gov, NCT06200727.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Highly myopic macular hole retinal detachment (MHRD) is associated with a low rate of macular hole (MH) closure and poor visual prognosis. |
Platelet-rich fibrin (PRF), an autologous blood derivative with established applications in regenerative medicine, demonstrates promise for promoting full-thickness MH healing. However, its efficacy in highly myopic MHRD remains unexplored. |
This study aimed to investigate the effectiveness of PRF membrane transplantation in highly myopic MHRD by comparing relevant metrics with the conventional approach of internal limiting membrane (ILM) peeling. |
What was learned from the study? |
Patients with highly myopic MHRD who underwent PRF treatment exhibited significantly improved visual acuity and a higher rate of MH closure compared to those who underwent ILM peeling. These findings suggest that PRF membrane transplantation offers a viable treatment option for highly myopic MHRD. |
The safety profile of the PRF membrane in the highly myopic MHRD cohort was deemed acceptable. No PRF-related complications were observed in the PRF-treated group, and neither group experienced serious complications. |
Digital Features
This article is published with digital features, including podcast audio, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.26180870.
Introduction
Highly myopic macular hole retinal detachment (MHRD) represents the terminal stage of complications arising from high myopia in the fundus [1]. The prevalence of MHRD is estimated to be approximately 1.01% in individuals with high myopia exceeding the age of 50, with a demonstrably higher incidence among female patients [2]. In cases of highly myopic MHRD, a significant decline in visual acuity is typically observed, necessitating prompt surgical intervention [3].
Highly myopic MHRD presents a significant therapeutic challenge due to several anatomical factors. These include a specific fundus base, limited internal limiting membrane (ILM) availability, and a non-planar retinal surface [4]. Despite the ongoing debate regarding the optimal treatment strategy for MHRD in highly myopic eyes, pars plana vitrectomy (PPV) combined with ILM peeling remains the most common approach. However, this technique yields an inconsistent macular hole (MH) closure rate, ranging from 25 to 88.9% [5, 6]. To improve outcomes, various novel surgical techniques have emerged since 2010, including the inverted ILM flap technique, initially proposed for large idiopathic MH and demonstrating a 98% closure rate [7]. Subsequently, modifications have been documented for highly myopic MHRD, such as the temporal inverted ILM flap technique [8], inverted ILM insertion [9], macular plug (inverted ILM flap insertion with blood coverage) [10], double or multiple free ILM flap insertion [11, 12], and autologous neurosensory retinal transplantation [13]. While these advancements have shown promise in improving MH closure and retinal reset rates, the evidence for their impact on best-corrected visual acuity (BCVA) compared to ILM peeling remains inconclusive, particularly regarding the ILM insertion methods [14, 15]. Furthermore, the range of potential materials for MH closure has expanded beyond the ILM to encompass lens capsular flaps (LCF) and amniotic membranes [16, 17]. Notably, a significant limitation in our current understanding is the predominance of retrospective and case-series studies in this area. Prospective randomized controlled trials are warranted to definitively assess the efficacy of these emerging techniques [16, 18].
Autologous blood derivatives, including platelet-rich plasma (PRP) [19] and plasma rich in growth factors [20], have been used for an extended period as adjuvants during MH surgery to promote closure [21, 22]. However, the application of these therapies has been limited by the complexity of their preparation process and the requirement for a relatively large blood volume (at least 9 ml) [23]. Platelet-rich fibrin (PRF), introduced by Choukroun et al. [24] as a second-generation platelet concentrate following PRP, offers a simpler and more cost-effective approach. PRF preparation utilizes only 5 ml of a patient’s venous blood, centrifuged without anticoagulants, resulting in a flexible solid that can be compressed into a membrane. This PRF membrane facilitates sustained and controlled release of growth factors, aiding tissue healing [25]. Due to these advantageous characteristics, PRF has gained widespread adoption across various surgical disciplines, including oral and maxillofacial surgery, and in broader regenerative medicine applications [26]. In the context of retinal diseases, PRF utilization remained limited until 2019, when Koytak et al. [27] reported successful closure of refractory MH in two patients treated with PRF. Our prior case series investigation also demonstrated a 100% MH closure rate and improved BCVA following PRF grafting for large MH [28]. These findings provide a compelling rationale for investigating the efficacy of PRF in the treatment of highly myopic MHRD.
A comprehensive review of the existing literature revealed no prior investigations into the application of PRF for the treatment of highly myopic MHRD. This study, therefore, aimed to evaluate the effectiveness of PRF membrane transplantation, in conjunction with silicone oil (SO) tamponade, compared to the established surgical approach of ILM peeling, in the management of patients with highly myopic MHRD.
Methods
Study Design
This investigation was designed as a randomized, prospective, and controlled clinical trial conducted at the Eye Center, Renmin Hospital of Wuhan University, China. The study protocol was registered at www.clinicaltrials.gov under the identifier NCT06200727 and received approval from the Clinical Research Ethic Committee of Renmin Hospital of Wuhan University, adhering to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participating patients.
Patients diagnosed with highly myopic MHRD were recruited between January 2022 and October 2023. In this study, participants undergoing PPV surgery were randomly allocated in a 1:1 ratio to either the conventional ILM peeling group (IP group) or the PRF membrane transplantation group (PMT group) using a simple randomization method with a table of random numbers. According to previous literature relevant to this study, MH closure rates of 93% and 50% were expected in the PMT and IP groups, respectively [6, 28, 29]. For the power calculation, assuming a power of 90% and a significance level (alpha) of 0.05, a sample size of 18 patients per group was determined. To account for 20% potential participant dropout, the total number of patients recruited per group was increased to 21. This resulted in a total of 42 patients enrolled in the study. All patients completed the follow-up period, except for two individuals in the IP group who withdrew from the study prematurely (Fig. 1).
Inclusion and Exclusion Criteria
This study recruited patients meeting the following inclusion criteria: (1) patients that were highly myopic between 18 and 90 years old, defined as having spherical equivalent refractive error of ≤ − 6.00 diopters and/or axial length (AL) ≥ 26.00 mm; (2) confirmed MHRD by optical coherence tomography (OCT); (3) minimal proliferative vitreoretinopathy (PVR) grade of less than D1; and (4) minimal MH diameter exceeding 400 µm.
Patients were excluded if they presented with: (1) combined peripheral retinal tears outside the macula; (2) coexisting ocular diseases such as glaucoma, uveitis, or diabetic retinopathy; (3) a history of previous fundus surgery; (4) serious underlying systemic co-morbidities; or (5) a positive preoperative evaluation for any infectious indicator.
Patient Assessment
Prior to surgery, all patients underwent a comprehensive preoperative evaluation encompassing a detailed medical history review, systemic examination, and ophthalmological assessment. The ophthalmological evaluation included measurement of BCVA in decimal units (converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis), intraocular pressure (IOP), AL using A/B-ultrasound (SW-2100, Tianjin Suowei Electronic Technology Co., Ltd, Tianjin, China), ultra-widefield scanning laser ophthalmoscopy (Daytona, Optos, Inc., Marlborough, MA, USA), and minimal MH diameter using OCT (AngioVue Optovue, Fremont, CA, USA). For patients with BCVA limited to counting fingers, hand movements, or light perception, logMAR values were assigned based on established literature: 1.85 for counting fingers, 2.3 for hand movements, and 2.6 for light perception [30]. Additionally, the Angio Retina QuickVue 3 mm × 3 mm mode of optical coherence tomography angiography (OCTA) (AngioVue Optovue, Fremont, CA, USA) was utilized to quantify whole superficial vascular density (SVD) and deep vascular density (DVD).
PRF Membrane Preparation
PRF membrane preparation adhered to the protocol established by Choukroun et al. [24]. Firstly, prior to entering the operating room, a 5-ml blood sample was obtained from the antecubital vein using a sterile blood collection tube devoid of anticoagulant or bovine thrombin. Secondly, the blood sample was then immediately centrifuged at 3000 rpm for 10 min using a table-top centrifuge (DMO421, Scilogex, Rocky Hill, CT, USA) on the ward (or in the operating room and laboratory where low-speed centrifuge is available). Centrifugation resulted in the formation of a PRF clot situated within the middle of the collection tube, positioned between the sedimented red corpuscles at the bottom and the acellular plasma layer at the top (Fig. 2a, b). Thirdly, employing sterile forceps within the operating room, the PRF clot was meticulously extracted from the red blood cell layer. The excess red blood cells were removed, retaining only the PRF fraction (Fig. 2c). Finally, the PRF clot was transferred to a PRF box (A17-0930, Pearson Dental, Sylmar, CA, USA) sterilized to operating room standards, and compressed for 5 min to yield the final PRF membrane (Fig. 2d, e). Throughout the entire procedure, rigorous principles of aseptic technique were maintained.
Preparation of the platelet-rich fibrin (PRF) membrane. a, b PRF is centrifuged into three layers, with an acellular plasma in the top-most layer, PRF clot in the middle, and a red corpuscle base at the bottom. c Cutting out excess red corpuscles. d The PRF box is compressing the PRF clot. e The PRF membrane is obtained after a 5 min-compression
Surgical Procedure
All surgeries were performed using a standard 23-gauge PPV system (Constellation Vision System, Alcon Laboratories, Inc., Fort Worth, TX, USA) by the same experienced vitreoretinal surgeon (Dr. Lei Du) under local anesthesia. Cataract surgery was performed when needed. Following complete removal of the posterior vitreous cortex or epiretinal membrane, subretinal fluid (SRF) was carefully drained from the MH. Subsequently, the ILM was stained with indocyanine green (ICG) (2.5 mg/ml, ICG, Dandong Yichuang Pharmaceutical Co. Ltd, Liaoning, China) for 30 s as an adjunct for visualization. In the IP group, the ILM was then completely removed within the arcade vessels, utilizing heavy water if necessary. Finally, after a thorough fluid–air exchange, SO was slowly injected into the vitreous cavity to achieve normal IOP.
In the PMT group, following ILM peeling to the margins of the arcade vessels, trimming of the PRF clot commenced (see Video 1 in the electronic supplementary material for details). Notably, either surface of the PRF graft was suitable for apposition with the RPE. To ensure stability during the subsequent fluid–air exchange, the PRF was trimmed into a plug-shaped implant. This implant possessed a slightly larger diameter at its superior aspect compared to the edge of the MH and narrower at the bottom. Additionally, gently cocking the MH edges using ILM forceps to create a subretinal space for graft placement can be employed, this step is optional. The PRF implant was then delivered atraumatically through the ILM forceps into the vitreous cavity and meticulously positioned onto the retinal surface in proximity to the MH. Subsequently, gentle pressure was applied to introduce the PRF into the MH, where the PRF implant could be held in the MH by the edge of the MH, thereby preventing displacement during the fluid–air exchange (Fig. 3). Notably, no instances of PRF displacement were observed intraoperatively within our patient cohort. Following a thorough fluid–air exchange, SO was injected into the vitreous cavity to achieve normal IOP. Postoperatively, all patients were instructed to maintain a prone positioning for a minimum duration of 1 month.
Surgical procedure for platelet-rich fibrin (PRF) membrane transplantation. a Staining of the internal limiting membrane (ILM) with indocyanine green (yellow arrows highlight the macular hole (MH)). b Peeling of the ILM using forceps. c Delivery of the PRF graft to the vitreous cavity (white arrows indicate the PRF membrane). d Placement of the PRF on the retinal surface near the MH. e Gentle pressure was applied to introduce the PRF into the MH. f The PRF graft remains stable during the fluid–air exchange
Outcome Measures
All patients were followed up postoperatively at 1 week, 1 month, 3 months, and 6 months. At each follow-up visit, patients underwent a comprehensive ophthalmological examination identical to the preoperative assessment. Notably, OCTA was only performed at the 6-month follow-up visit. The primary outcome measure of this study was to compare the rates of MH closure at the 6-month follow-up. The final statistical analysis was conducted by comparing preoperative data with data collected at the 6-month follow-up visit. Secondary outcome measures included the assessment and comparison of BCVA, central retinal thickness (CRT), rate of retinal reattachment, SVD, DVD, and any complications encountered during the study period.
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). Intergroup comparisons for continuous variables were conducted using unpaired Student’s t tests. Categorical variables were analyzed using chi-squared tests or Fisher’s exact tests, as appropriate, based on sample size considerations. To evaluate changes in BCVA and CRT within each group over time, a repeated-measures analysis of variance (ANOVA) was employed. Significant main effects from the ANOVA were followed by post hoc comparisons using the Bonferroni correction for multiple comparisons. A two-tailed P value of less than 0.05 was considered statistically significant.
Results
Forty patients (40 eyes) completed the 6-month postoperative follow-up, with 19 eyes in the IP group and 21 eyes in the PMT group. Baseline demographic characteristics (age, gender, and operated eye), along with ophthalmic parameters including IOP, BCVA, CRT, lens status, the time to SO removal post-surgery, and the observation period following SO removal, were comparable between the two groups (Table 1).
Anatomical Outcomes
MH closure and retinal reset. The primary outcome measure of this study was the rate of MH closure at the 6-month follow-up visit. The mean follow-up duration for the IP group was 6.11 ± 0.17 months, and for the PMT group, it was 6.17 ± 0.11 months (P = 0.230). The closure rate in the IP group was 78.95% (15 of 19 eyes) at 6 months, while the PMT group achieved a closure rate of 100.00% (21 of 21 eyes). This significant difference between groups was observed with a p value of 0.042 (detailed results presented in Figs. 4 and 5). Notably, all patients experienced retinal reattachment, regardless of the treatment group (Table 2). Of the four individuals in the IP group who did not achieve MH closure, two patients discontinued further treatment for personal reasons, while the other two are currently undergoing treatment with PRF membrane and are being monitored. Although the MH closure rate in the IP group did not reach 100%, complete retinal reattachment was achieved in all cases (Fig. 6).
Fundus photographs and optical coherence tomography (OCT) images following platelet-rich fibrin (PRF) membrane transplantation and silicone oil tamponade for macular hole retinal detachment (MHRD) in a 53-year-old male with high myopia. a, b Preoperative fundus photograph and OCT image show MHRD and the minimum diameter of macular hole (MH) is 1030 µm. The blue boxed line indicates the location of the OCT raster. c, d 1-week follow-up fundus and OCT photograph showed that retinal reset and unabsorbed PRF membrane (as indicated by yellow arrow). New tissue formation (white arrow) is bridging the edges of the MH on the PRF graft. e, f Fundus photograph and OCT image at 1 month show a closed MH. g, h A 3-month follow-up fundus photograph and OCT image. i, j A 6-month follow-up fundus photograph and OCT image
Follow-up images of a 55-year-old female patient with macular hole retinal detachment (MHRD) in highly myopic after internal limiting membrane (ILM) peeling and silicone oil tamponade. a, b Preoperative fundus photograph and optical coherence tomography (OCT) image showing MHRD and the minimum diameter of macular hole (MH) was 536 µm. The blue boxed line indicates the location of the OCT raster. c, d 1-week follow-up fundus and OCT photograph showing a reattached retina with exposed retinal pigment epithelium (RPE) layer (indicated by the white arrows). e, f Fundus photograph and OCT image at 1-month follow-up depict persistent exposure of the RPE layer. g, h Fundus photograph and OCT image at 3-month follow-up demonstrate slight improvement in the exposed RPE layer. i, j Fundus photograph and OCT image at 6-month follow-up show retinal reattachment and closure of the MH
Preoperative and 6-month postoperative images of a 58-year-old highly myopic female with macular hole retinal detachment (MHRD) who underwent internal limiting membrane peeling and silicone oil tamponade. a The preoperative optical coherence tomography (OCT) image revealed the presence of MHRD and a macular hole (MH) with a minimum diameter of 463 µm. The blue triangle indicates the location of the OCT raster. b The OCT image taken 6-month follow-up revealed that the retina had reset at the macula, but the minimum diameter of the MH had expanded to 1200 µm. c The preoperative fundus photograph showed MHRD. d Fundus photograph taken 6-month follow-up depicted a complete resolution of the retinal detachment
CRT alteration. CRT measurements were consistently higher in the PMT group compared to the IP group at all postoperative follow-up visits (Table 3). Statistically significant differences were observed at 1 week (P < 0.001), 3 months (P = 0.002), and 6 months (P = 0.002) postoperatively. Notably, CRT did not differ significantly between the groups at the 1-month follow-up visit (P = 0.214). Over the follow-up period, both groups demonstrated a significant decrease in CRT compared to baseline measurements (P < 0.001 for all time points) (Fig. 7a). This rapid decrease in CRT was evident in both groups at 1 week. The IP group exhibited a minimum CRT value of 171.16 ± 4.39 µm at 3 months, whereas the PMT group achieved a minimum CRT value of 179.00 ± 3.15 µm at 1 month.
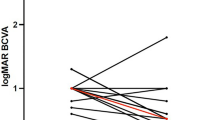
Time profile of central retinal thickness (CRT) (a) and best-corrected visual acuity (BCVA) (b) in the internal limiting membrane peeling (IP) group and platelet-rich fibrin membrane transplantation (PMT) group. Statistical comparisons between preoperative baseline and postoperative follow-up time points were made using one-way repeated measures analysis of variance (ANOVA) with Bonferroni post hoc test for multiple comparisons. Asterisks (*) and number signs (#) indicate significant differences in the IP and PMT groups, respectively, compared to the preoperative baseline. Significance levels: **P < 0.01; ***P < 0.001; ### P < 0.001. Pre, preoperative; 1w, 1-week follow-up;1 m, 1-month follow-up; 3 m, 3-month follow-up; 6 m, 6-month follow-up
Retinal vascular density outcomes. At the 6-month follow-up, the PMT group demonstrated significantly higher mean SVD compared to the IP group (39.16 ± 5.18% vs. 43.21 ± 2.32%, P = 0.002). Similarly, the PMT group exhibited a significantly higher mean DVD compared to the IP group (41.97 ± 4.86% vs. 45.55 ± 3.75, P = 0.013) (Table 2).
Visual Outcomes
In both groups, statistically significant differences in BCVA were observed across all postoperative time points when compared to baseline (P < 0.01), with the exception of one week postoperatively in the IP group (P = 0.346, Fig. 7b). No significant difference in BCVA was found between the two groups at 1 week postoperatively (P = 0.203, Table 3). However, BCVA in the PMT group was significantly higher compared to the IP group at 1 month postoperatively (P = 0.032). This trend of superior BCVA in the PMT group persisted at 3 months (P = 0.025) and 6 months (P = 0.040) postoperatively.
Safety Outcomes
Several complications were observed during the follow-up period. These included IOP changes, corneal edema, and SRF. All instances were resolved or normalized following appropriate symptomatic treatment. SO emulsification and cataract formation were addressed through SO removal and cataract extraction surgery. Notably, there were no statistically significant differences in the frequency of complications between the two groups (Table 4). Regarding the PRF membrane grafts, complete absorption was observed within the 6-months follow-up period. In this respect, at the 1-week follow-up, none of the grafts displayed complete dissolution. However, by the 1-month follow-up, 14 grafts had completely dissolved, with this number increasing to 19 grafts by the 3-month follow-up. Importantly, no serious complications such as endophthalmitis, vitreous hemorrhage, or choroidal bleeding, were encountered in either group during or after surgery.
Discussion
This randomized controlled trial investigated a novel therapeutic approach for treating MHRD in patients that were highly myopic. The study compared the effectiveness of PRF membrane transplantation with the established surgical technique of ILM peeling. Postoperative anatomical and visual function outcomes, as well as complication rates, were assessed in both groups.
While research on the application of blood products for treating MH with retinal detachment in highly myopic eyes remains limited compared to non-detached MH, existing studies using blood products for MH treatment offer promising results. Notably, investigators have explored the use of inverted ILM flap insertion combined with blood coverage to manage MHRD in that were highly myopic, achieving improved MH closure rates and BCVA [31, 32]. These findings suggest that blood-derived growth factors potentially promote hole closure, and blood products may mitigate the potential toxic effects of ICG on retinal tissue [33]. However, a risk associated with autologous blood use is leakage into the subretinal space before coagulation. PRF, a solid blood derivative, offers advantages in terms of simpler preparation and potentially improved safety compared to whole blood. Consequently, PRF transplantation presents a potentially superior alternative to the ILM peeling, insertion, and covering technique by eliminating the potential for ICG-related retinal toxicity and minimizing the risk of anterior retinal fluid draining into the sub-retina.
A recent prospective interventional case series investigated the use of PRP injection for the treatment of eyes with MH and RD [34]. Although the study demonstrated consistent MH closure throughout the 2-year follow-up period, one eye experienced recurrent RD due to the reopening of a previously PRP-blocked retinal tear. Interestingly, Parisi et al. [35] reported a similar case of coexisting recurrent RD with persistent MH closure following PRP injection. These findings raise concerns about a potential mechanism by which PRP may induce biomechanical alterations at the retinal level. The injection of PRP into the MH as a liquid may result in the adherence of the PRP to the opposing edges of the MH during the subsequent solidification process, thereby creating a tensile force. In this context, speculation exists that PRP-mediated contraction of the MH could inadvertently propagate traction forces to the peripheral retina, thereby increasing the risk of secondary retinal tears.
In the present study, the PMT group achieved a 100% rate of MH closure. The IP group achieved a retinal reattachment rate of 100% and an MH closure rate of 78.95%, which fell within the upper-middle range reported in previous studies using conventional ILM peeling [5, 36]. Notably, there were no instances of MH reopening, peripheral retinal breaks, or RD recurrence observed in the PRF-treated group during the follow-up period. One potential mechanism for the observed efficacy of PRF may be related to the solidification process. Unlike liquid PRP, PRF does not contain anticoagulants and forms a solid structure after centrifugation. This PRF membrane was then trimmed and molded into a plug shape specifically designed to fit the dimensions of the MH, with a wider top and a narrower base. This configuration allows the PRF to contact, rather than adhere to, the opposing edges of the MH. In the PMT group, we observed new tissue initiating MH closure by migrating across the PRF scaffold from both ends. The solid PRF structure is composed of thick fibrin bundles with a rough surface, creating a complex fibrin network that serves as a scaffold for MH healing. Additionally, the fibrin network contains a significant number of platelets, a smaller number of leukocytes, and circulating stem cells. These components are known to steadily release a variety of cytokines and growth factors, potentially promoting the proliferation and migration of Müller cells [37, 38]. Ultimately, the PRF membrane is gradually absorbed and replaced by retinal tissue. While the precise mechanisms underlying PRF-mediated MH healing require further investigation through basic science experiments, it is possible that in highly myopic retinas experiencing high levels of tension, the specific properties and placement of the PRF, combined with its resorption characteristics, may not exacerbate retinal traction, potentially reducing the risk of peripheral retinal tears.
In highly myopic eyes, the combination of a long AL and a retinal architecture that is deficient in resilience can lead to a compromised adhesion between the retinal neuroepithelial layer and the RPE layer. This can manifest as an MH, which allows fluid from the vitreous cavity to pass through and accumulate subretinally, separating the neuroepithelium from the RPE and forming MHRD [1]. Consistent with these pathological mechanisms, both groups exhibited elevated CRT values preoperatively.
Throughout the follow-up period, a pattern of decreasing CRT was observed in both groups. Notably, the CRT demonstrated a rapid decline during the first postoperative week compared to baseline. This initial decrease was followed by a sustained reduction, with the CRT in the IP group reaching its nadir before a gradual rise beginning in the third postoperative month. The PMT group exhibited a similar pattern, with the CRT reaching its lowest point and beginning to increase 1 month postoperatively. To our knowledge, this specific postoperative CRT trend has not been previously reported in the literature for patients with MHRD. While the precise mechanisms underlying this observed trend remain unclear, we posit that the findings may be partially explained by the “hydration theory” proposed by Tornambe et al. [39]. In MHRD, exposure of the retina to vitreous fluid can lead to retinal edema and a resultant increase in CRT. Following surgery, the vitreous is replaced with SO, and the PRF membrane in the PMT group serves as a physical barrier, effectively eliminating the SRF and leading to a rapid decrease in CRT. However, a potential delay in the resolution of retinal edema could contribute to the continued decrease in CRT as edema subsides. Additionally, in the PMT group, the gradual absorption of the PRF graft may also play a role in the observed CRT changes. Ultimately, as the MH heals, the CRT is expected to gradually return towards normal values.
During the course of retinal reset and MH closure, there was retinal vascular remodeling, metabolic restoration, and resorption of perifoveal cystoid cavities. These processes culminated in the progressive recovery of both blood perfusion and BCVA [40, 41]. However, preoperative assessment of blood flow density using OCTA may be hindered by the presence of macular detachment and significantly reduced visual acuity [42]. This limitation persists despite efforts to mitigate it, such as excluding poor-quality images, employing the whole blood flow density measurements within the designated area, and, in some cases, performing multiple measurements. Caporossi et al. [43] demonstrated a strong correlation between postoperative blood flow density and BCVA in patients with highly myopic MHRD. In our study, with all 40 patients achieving retinal reintegration, we compared the blood flow density between the two groups at 6 months postoperatively. We observed that both SVD and DVD were significantly higher in the PMT group compared to the IP group, potentially signifying the benefit of PRF treatment in terms of retinal blood flow density restoration.
While no PRF-related complications were observed in our study, a discussion of potential adverse events is warranted. PRF contains leukocytes that secrete cytokines, which not only regulate cell growth but also release inflammatory mediators, including interleukin-1β, interleukin-6, tumor necrosis factor-α, and vascular endothelial growth factor [44]. These factors play a complex role, exhibiting both anti-infective properties and promoting tissue healing through modulation of inflammation and angiogenesis [45, 46]. However, excessive inflammation and neovascularization are potential complications associated with PRF use. PRF exhibits a relatively short resorption time. The transient nature of PRF within the fundus may explain the absence of these complications in our study. In a comparable investigation, Chen et al. [47] employed an amniotic membrane as an inlay for MH repair in patients that were highly myopic. They reported that the amniotic membrane persisted for up to 12 months postoperatively without compromising BCVA recovery. Conversely, the biodegradability of LCF grafts remains unestablished [17, 48, 49]. Our findings demonstrated a 90.48% resorption rate of PRF by the third month postoperatively, with complete resorption observed in all eyes by 6 months. These results confirm the intraocular absorption of PRF, the absorption property corroborated by studies in various other disciplines [50, 51].
PRF preparation is a straightforward process, and the subsequent surgical procedure imposes less technical demands on the surgeon. Following centrifugation, the PRF clot can be conveniently compressed into a membrane form between two sterile gauze pads, eliminating the need for specialized PRF processing tools. A crucial step involves trimming the PRF membrane into a plug-shaped implant. This implant should be slightly larger than the MH at the top and slightly narrower at the bottom, thereby reducing the probability of displacement during fluid–air exchange. The ample volume of the prepared PRF allows for repeated trimming to achieve the desired configuration. Notably, PRF offers a valuable surgical modality for the management of challenging cases, including: (1) Recurrent MHRD, highly myopic MHRD, and Alport syndrome, where ILM acquisition may be particularly difficult [52]. (2) LCF transplantation is not a feasible option in cases where there is no anterior LCF and calcification of the posterior LCF in intraocular lens eyes. (3) Amniotic membrane transplantation is not a financially viable option. However, rigorous preoperative pathogen screening and meticulous aseptic technique are paramount throughout the procedure.
Our study is subject to several limitations that warrant consideration. First, the sample size employed was relatively modest, and the follow-up period was brief. It is well established that the restoration of visual function and anatomical features in MHRD can be a protracted process, and this extended timeframe was not fully captured within the scope of this investigation. Second, the study design focused solely on the assessment of BCVA, neglecting a comprehensive evaluation of visual function. An analysis of retinal sensitivity using techniques such as electroretinography and microperimetry was not incorporated. Third, while PPV combined with ILM peeling represents the current standard surgical approach, the variable rate of MH closure presents a challenge in definitively attributing all observed benefits to the ILM peeling procedure. It is possible that a simple PPV with ILM peeling may not preserve the benefits of the patients in the IP group. Future studies may benefit from including an intervention arm where ILM is inserted or transplanted, or where other materials are employed, as a control group. Finally, it is important to acknowledge that the generalizability of our findings may be limited, as all surgeries were performed by a single, highly experienced physician. The outcomes achieved by less experienced surgeons were not evaluated within this study design.
Conclusions
In summary, this study demonstrates the efficacy and safety of PRF membrane transplantation for treating MHRD in patients that were highly myopic. Compared to conventional surgical ILM peeling, PRF transplantation resulted in superior outcomes in terms of MH closure rates, BCVA, retinal thickness, and retinal blood flow. Notably, this approach was not associated with any additional complications. To further solidify these promising findings, future multicenter randomized controlled trials with larger patient cohorts, extended follow-up durations, and more comprehensive ophthalmic examinations are warranted.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to internal rules and privacy issues.
References
Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, García-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80–115.
Chen Q, He J, Hu G, Xu X, Lv H, Yin Y, et al. Morphological characteristics and risk factors of myopic maculopathy in an older high myopia population-based on the new classification system (ATN). Am J Ophthalmol. 2019;208:356–66.
Li KKW, Wong DHT, Li PSH. Are we facing an increasing surgical demand for high myopic traction maculopathies? A 12-year study from Hong Kong. BMC Ophthalmol. 2023;23(1):31.
Jonas JB, Jonas RA, Bikbov MM, Wang YX, Panda-Jonas S. Myopia: histology, clinical features, and potential implications for the etiology of axial elongation. Prog Retin Eye Res. 2023;96: 101156.
Wang ZQ, Ni ZZ, Zhang XL, Lin XY, Hu XT, Zhang ZL, et al. Vitrectomy for retinal detachment associated with macular hole: prognostic factor analysis under different axial length conditions. Acta Ophthalmol. 2023;102(4):e557–64.
Xu Q, Luan J. Vitrectomy with inverted internal limiting membrane flap versus internal limiting membrane peeling for macular hole retinal detachment in high myopia: a systematic review of literature and meta-analysis. Eye (Lond). 2019;33(10):1626–34.
Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–25.
Ho TC, Ho A, Chen MS. Vitrectomy with a modified temporal inverted limiting membrane flap to reconstruct the foveolar architecture for macular hole retinal detachment in highly myopic eyes. Acta Ophthalmol. 2018;96(1):e46–53.
Chen SN, Yang CM. Inverted internal limiting membrane insertion for macular hole-associated retinal detachment in high myopia. Am J Ophthalmol. 2016;162:99-106.e1.
Wu AL, Ling KP, Chuang LH, Chen KJ, Chen YP, Yeung L, et al. Treatment of macular hole retinal detachment with macular plug in highly myopic eyes: three-year results. Acta Ophthalmol. 2020;98(7):e839–47.
Chen SN, Yang CM. Double internal limiting membrane insertion for macular hole-associated retinal detachment. J Ophthalmol. 2017;2017:3236516.
Chen SN, Hsieh YT, Yang CM. Multiple free internal limiting membrane flap insertion in the treatment of macular hole-associated retinal detachment in high myopia. Ophthalmologica. 2018;240(3):143–9.
Moysidis SN, Koulisis N, Adrean SD, Charles S, Chetty N, Chhablani JK, et al. Autologous retinal transplantation for primary and refractory macular holes and macular hole retinal detachments: the global consortium. Ophthalmology. 2021;128(5):672–85.
Chen Y, Wang J, Ye X, Yu J, Tao J, Lin L, et al. The role of internal limiting membrane flap for highly myopic macular hole retinal detachment: improving the closure rate but leading to excessive gliosis. Front Med (Lausanne). 2021;8: 812693.
Chatziralli I, Machairoudia G, Kazantzis D, Theodossiadis G, Theodossiadis P. Inverted internal limiting membrane flap technique for myopic macular hole: a meta-analysis. Surv Ophthalmol. 2021;66(5):771–80.
Caporossi T, De Angelis L, Pacini B, Tartaro R, Finocchio L, Barca F, et al. A human Amniotic Membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2020;98(2):e252–6.
Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–70.
Chen YC, Yang CM, Chen SN. Lens capsular flap in the management of posterior retinal hole associated retinal detachment in high myopic eyes with previous internal limiting membrane peeling: 3 case reports. Medicine (Baltimore). 2019;98(29): e16422.
Gehring S, Hoerauf H, Laqua H, Kirchner H, Klüter H. Preparation of autologous platelets for the ophthalmologic treatment of macular holes. Transfusion. 1999;39(2):144–8.
Arias JD, Hoyos AT, Alcántara B, Sanchez-Avila RM, Arango FJ, Galvis V. Plasma rich in growth factors for persistent macular hole: a pilot study. Retin Cases Brief Rep. 2022;16(2):155–60.
Liggett PE, Skolik DS, Horio B, Saito Y, Alfaro V, Mieler W. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology. 1995;102(7):1071–6.
Cao JL, Kaiser PK. Surgical management of recurrent and persistent macular holes: a practical approach. Ophthalmol Ther. 2021;10(4):1137–53.
Baca-Gonzalez L, Serrano Zamora R, Rancan L, González Fernández-Tresguerres F, Fernández-Tresguerres I, López-Pintor RM, et al. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): comparative release of growth factors and biological effect on osteoblasts. Int J Implant Dent. 2022;8(1):39.
Choukroun JAF, Schoeffler C, Vervelle A. Une opportunite´ en paro-implantologie: le PRF. Implantodontie. 2000;42:55–62.
Wang X, Fok MR, Pelekos G, Jin L, Tonetti MS. In vitro and ex vivo kinetic release profile of growth factors and cytokines from leucocyte- and platelet-rich fibrin (L-PRF) preparations. Cells. 2022;11(13):2089.
Giannotti L, Di Chiara SB, Spedicato F, Nitti P, Damiano F, Demitri C, et al. Progress in regenerative medicine: exploring autologous platelet concentrates and their clinical applications. Genes (Basel). 2023;14(9):1669.
Koytak A, Nuhoglu F, Bayraktar H, Ercan R, Ozdemir H. Autologous platelet-rich fibrin in the treatment of refractory macular holes. Case Rep Ophthalmol Med. 2019;2019:6054215.
Yang N, Zeng S, Yang J, Lu G, Du L. Application of platelet-rich fibrin transplantation for large macular hole. Curr Eye Res. 2022;47(5):770–6.
Takahashi H, Inoue M, Koto T, Itoh Y, Hirota K, Hirakata A. Inverted internal limiting membrane flap technique for treatment of macular hole retinal detachment in highly myopic eyes. Retina. 2018;38(12):2317–26.
Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg Visual Acuity Test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–40.
Lai CC, Chen YP, Wang NK, Chuang LH, Liu L, Chen KJ, et al. Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 2015;122(9):1889–98.
Ying HF, Wu SQ, Hu WP, Ni LY, Zhang ZL, Xu YG. Vitrectomy with residual internal limiting membrane covering and autologous blood for a secondary macular hole: a case report. World J Clin Cases. 2022;10(2):671–6.
Li Y, Li Z, Xu C, Liu Y, Kang X, Wu J. Autologous neurosensory retinal transplantation for recurrent macular hole retinal detachment in highly myopic eyes. Acta Ophthalmol. 2020;98(8):e983–90.
Malyugin BE, Shkvorchenko DO, Khurdaeva AG. Long-term outcomes of treating rhegmatogenous retinal detachment with macular hole using platelet-rich blood plasma. Vestn Oftalmol. 2023;139(2):6–10.
Parisi G, Ricardi F, Boscia G, Ghilardi A, Gelormini F, Marolo P, et al. Macula-off retinal detachment with refractory macular hole previously closed with autologous platelet-rich plasma: a case report. Case Rep Ophthalmol. 2023;14(1):462–8.
Liu X, Huang J, Zhou R, Jiang Z, Chen H, Chen W, et al. Comparison of internal limiting membrane peeling with the inverted internal limiting membrane flap technique for rhegmatogenous retinal detachment coexisting with macular hole. Retina. 2022;42(4):697–703.
Bai MY, Wang CW, Wang JY, Lin MF, Chan WP. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clinics (Sao Paulo). 2017;72(2):116–24.
Wu AL, Liu YT, Chou HD, Chuang LH, Chen KJ, Chen YP, et al. Role of growth factors and internal limiting membrane constituents in Müller cell migration. Exp Eye Res. 2021;202: 108352.
Tornambe PE. Macular hole genesis: the hydration theory. Retina. 2003;23(3):421–4.
Savastano A, Bacherini D, Savastano MC, Finocchio L, Dragotto F, Lenzetti C, et al. Optical coherence tomography angiography findings before and after vitrectomy for macular holes: useful or useless? Retina. 2021;41(7):1379–88.
Cicinelli MV, Marchese A, Bandello F, Coppola M. Inner retinal layer and outer retinal layer findings after macular hole surgery assessed by means of optical coherence tomography. J Ophthalmol. 2019;2019:3821479.
Hong EH, Cho H, Kim DR, Kang MH, Shin YU, Seong M. Changes in retinal vessel and retinal layer thickness after vitrectomy in retinal detachment via swept-source oct angiography. Invest Ophthalmol Vis Sci. 2020;61(2):35.
Caporossi T, Governatori L, Gambini G, Baldascino A, De Vico U, Ripa M, et al. Treatment of recurrent high myopic macular hole associated with retinal detachment using a human amniotic membrane. Jpn J Ophthalmol. 2022;66(6):518–26.
Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473(5):1635–43.
Ozcan M, Kabaklı SC, Alkaya B, Isler SC, Turer OU, Oksuz H, et al. The impact of local and systemic penicillin on antimicrobial properties and growth factor release in platelet-rich fibrin: in vitro study. Clin Oral Investig. 2023;28(1):61.
Zumstein MA, Rumian A, Lesbats V, Schaer M, Boileau P. Increased vascularization during early healing after biologic augmentation in repair of chronic rotator cuff tears using autologous leukocyte- and platelet-rich fibrin (L-PRF): a prospective randomized controlled pilot trial. J Shoulder Elbow Surg. 2014;23(1):3–12.
Chen H, Lin W, Tang Y, Wei Y. The long-term follow-up of autologous blood clot-assisted lyophilized human amniotic membrane covering treatment unclosed macular hole. Retina. 2023;43(8):1340–7.
Peng J, Chen C, Zhang L, Huang Y, Zhang H, Zheng Y, et al. Lens capsular flap transplantation as primary treatment for closure of large macular holes. Retina. 2022;42(2):306–12.
Peng J, Chen C, Jin H, Zhang H, Zhao P. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina. 2018;38(11):2177–83.
Hama S, Yokoi T, Orita K, Uemura T, Takamatsu K, Okada M, et al. Peripheral nerve regeneration by bioabsorbable nerve conduits filled with platelet-rich fibrin. Clin Neurol Neurosurg. 2023;236: 108051.
Polak D, Falcoff D, Chachartchi T, Asher R, Assad R. Sustainability and release pattern of growth factors from bone grafts prepared with platelet-rich fibrin. Int J Oral Maxillofac Implants. 2024;21(3):473–478.
Chaudhry SG, Liew G, Fung AT. Missing internal limiting membrane during macular hole repair in Alport syndrome. Case Rep Ophthalmol. 2021;12(2):320–3.
Acknowledgements
We would like to thank all study participants for their involvement in the study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding
The study and journal’s Rapid Service Fee were funded by the China Primary Health Care Foundation (No. [2022] Year [005]).
Author information
Authors and Affiliations
Contributions
Research design: All the authors. Patient enrollment and surgical treatment: Lei Du, Guojing Lu, Rong Huang; Data collection: Guojing Lu, Lei Du, Siyu Zeng. Data analysis: Guojing Lu, Siyu Zeng. Manuscript preparation: Guojing Lu, Lei Du. Manuscript review: Lei Du, Rong Huang.
Corresponding authors
Ethics declarations
Conflict of Interest
Guojing Lu, Siyu Zeng, Rong Huang, and Lei Du confirm that they have no conflicts of interest to declare.
Ethical Approval
This prospective, randomized-control study (NCT06200727) has been approved by the Clinical Research Ethic Committee, Renmin Hospital of Wuhan University (ID: WDRY2022-K197). All subjects provided informed consent to participate in the study, which was conducted according to the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 129784 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lu, G., Zeng, S., Huang, R. et al. Platelet-rich Fibrin Membrane Transplantation for the Treatment of Highly Myopic Macular Hole Retinal Detachment. Ophthalmol Ther (2024). https://doi.org/10.1007/s40123-024-00997-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40123-024-00997-w