Abstract
Introduction
This study evaluated 10-year results of implanting one iStent trabecular micro-bypass stent during cataract surgery in eyes with open-angle glaucoma (OAG) or ocular hypertension.
Methods
This retrospective, non-randomized study examined 10-year outcomes of iStent trabecular micro-bypass stent implantation with cataract surgery by one surgeon in eyes with OAG [including primary OAG (POAG) and pseudoexfoliative glaucoma (PXG)] or ocular hypertension at a multi-specialty German ophthalmology center. Study visits were conducted preoperatively and at 2.5, 3, 5, and 10 years postoperatively; examinations included intraocular pressure (IOP), medications, corrected-distance visual acuity (CDVA), and adverse events.
Results
A total of 63 eyes of 45 patients with OAG (n = 60 eyes) or ocular hypertension (n = 3 eyes) and data through 10 years were analyzed. Mean preoperative IOP was 18.6 ± 4.4 mmHg on 1.83 ± 1.03 mean medications. At study visits through 10 years postoperative, mean IOP reduced by 12.9–19.0% (p < 0.005 at all points), and mean medication burden reduced by 37.8–51.4% (p ≤ 0.006 at all points). At 10 years postoperatively, 77.8% of eyes had IOP ≤ 18 mmHg and 47.6% had IOP ≤ 15 mmHg (vs. 50.8% and 25.4% preoperatively, respectively; p = 0.016). One-third (33.3%) of eyes were medication-free vs. 3.2% preoperatively (p < 0.001); 17.5% were on 2–5 medications (vs. 55.6% preoperatively, p = 0.005); and 93.7% of eyes were on the same or fewer medications vs. preoperative. Post-phacoemulsification CDVA improvement was maintained; no filtering surgeries were completed over 10-year follow-up.
Conclusions
Significant and safe IOP and medication reductions were observed through 10 years after iStent implantation with cataract surgery in patients with OAG or ocular hypertension.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Why carry out this study? |
First-generation trabecular micro-bypass (iStent) implantation has been shown to be safe and effective over the short and long term, with outcomes published through up to 8 years postoperative. |
Prior studies have been done in all severities of glaucoma and ocular hypertension, and in both combined and standalone settings. |
The present 10-year study is the longest-running analysis of trabecular micro-bypass outcomes to-date, with data reported in a real-world generalizable patient setting that may inform surgeons’ clinical practice. |
What was learned from the study? |
First-generation trabecular micro-bypass stent implantation with cataract surgery resulted in significant and safe reductions in intraocular pressure and medications through 10 years postoperatively in eyes with glaucoma and ocular hypertension. |
The outcomes provide real-world evidence of the safety and efficacy of trabecular micro-bypass over time in eyes with glaucoma and cataract. |
Introduction
Glaucoma is the leading cause of global irreversible blindness, posing a significant public health burden. Traditional treatment paradigms have often relied on extended treatment with topical medications, followed by watchful waiting until an invasive filtering surgery such as trabeculectomy or tube shunt implantation is needed. However, under this paradigm, treated patients still too often progress to blindness [1, 2]. In recent years, as minimally invasive surgical interventions have become more widespread, this traditional topical-medications-first paradigm has come under increased scrutiny. Surgeons are increasingly recognizing the limitations of topical medications, together with the benefits of earlier more proactive surgical intervention in the disease process—a paradigm shift that has been termed interventional glaucoma [3, 4].
The glaucoma treatment paradigm is indeed evolving. Laser trabeculoplasty is now accepted as a viable first-line therapy, bolstered by the findings of the Laser in Glaucoma and Ocular Hypertension (LiGHT) trial [5]. Micro-invasive glaucoma surgery (MIGS) is also widely available and utilized, growing to account for the majority of all glaucoma surgeries in the US [6]. This rise in MIGS has been accompanied by a concomitant decline in traditional filtering surgeries [7]. A third area of recent development has been intracameral procedural pharmaceuticals such as the bimatoprost intracameral implant (Durysta®, Allergan, Irvine, CA) and the sustained-release iDose® TR travoprost intraocular implant (Glaukos, Aliso Viejo, CA, USA).
In the realm of MIGS, the longest-term study to date was published by Salimi et al. [8] who evaluated 8-year outcomes of implanting two iStent trabecular micro-bypass stents in combination with cataract surgery. The study showed significant reductions in intraocular pressure and medications through 8 years postoperative, with favorable safety including a low incidence (1/62 or < 2%) of secondary filtering surgery. In the present paper, we evaluate 10-year outcomes of single-iStent implantation with phacoemulsification in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) in a single private practice in Germany. To our knowledge, this is the longest-running MIGS study to date, providing real-world evidence of the safety and efficacy of the procedure over time.
Methods
Study Design, Participants, and Endpoints
This retrospective consecutive case series included all iStent cases (either with or without phacoemulsification) completed by a single surgeon over a 4-year period at a private ophthalmology center in Munich, Germany. Inclusion criteria were as follows: diagnosis of OAG [including primary OAG (POAG) and pseudoexfoliative glaucoma (PXG)] or ocular hypertension (OHT); cataract requiring surgery (for combined cases); need for intraocular pressure (IOP) and/or medication reduction; and IOP and medication data available at the preoperative and 10-year visits. Similar to the other two prior publications from our practice, patients’ preoperative glaucoma severity was not categorized according to an external metric or scale. Rather, a holistic patient-centric quantification of glaucoma disease burden was used, based upon preoperative IOP and number of medications. Eyes with IOP ≤ 18 mmHg and ≤ 1 medication were categorized as “light” burden;” eyes with either IOP > 18 mmHg OR ≥ 2 medications had “medium” burden; and eyes with IOP > 18 mmHg and ≥ 2 medications were categorized as “heavy” burden. Cataract surgery was indicated for a drop of visual acuity below the legal limit for the German driving license: worse than 20/40 vision in the better eye and worse than 20/60 vision in the second eye. Patients with intumescent or rubra cataracts were not included in the study. Eyes could be included regardless of prior surgical history, preoperative IOP, or preoperative medication burden. Exclusion criteria consisted of angle closure, active intraocular inflammation, and angle abnormalities or corneal opacities precluding intraoperative gonioscopy. Prior iterations of this dataset were published after 3 years and 5 years of follow-up [9, 10], but due to differing lengths of follow-up and numbers of available eyes, the present dataset is analyzed as separate from—rather than a continuation of—these prior cohorts. Patient visits and subsequent data analyses abided by the Declaration of Helsinki and the ethical requirements of the relevant committee on human research (Bayrische Landesärztekammer München). All patients gave informed consent prior to undergoing surgery.
Effectiveness outcomes included mean IOP and number of ocular hypotensive medications, proportions of eyes with IOP ≤ 18 mmHg or ≤ 15 mmHg, proportions of eyes on no medications or ≥ 2 medications, and proportion of eyes on 0/1/2/3/4/5 medications. Safety evaluations included corrected-distance visual acuity (CDVA), adverse events, and secondary glaucoma surgeries through 10 years postoperative. CDVA was analyzed for the overall cohort as well as excluding eyes with age-related macular degeneration or optic atrophy. As in the prior publications from this site [9, 10], detailed preoperative visual field (VF) data were not part of the implantation criteria, and preoperative and postoperative VF data were not available for all patients in the study; thus CDVA served as a surrogate of visual function.
Trabecular Micro-Bypass Stent, Surgical Technique, and Postoperative Medication
After standard phacoemulsification cataract extraction and intraocular lens implantation, a single first-generation iStent trabecular micro-bypass stent was advanced ab internally to the nasal anterior chamber angle through the same temporal clear corneal incision used during phacoemulsification. The stent was then implanted through the trabecular meshwork and into Schlemm’s canal. As described in prior publications [9, 11], the iStent is a single-piece L-shaped biocompatible titanium stent that is preloaded on a single-use stainless-steel inserter. The stent is designed to decrease IOP by increasing trabecular outflow from the anterior chamber to Schlemm’s canal.
Immediately following stent implantation, two drops of topical antibiotic (ofloxacin) were instilled. Postoperatively, patients were prescribed topical anti-inflammatory medication (bromfenac twice daily) for 3 weeks. Topical corticosteroid drops were not used postoperatively, as in the surgeon’s experience they were not necessary given the minimal levels of inflammation induced by the surgery. Patients’ preoperative topical glaucoma medication was discontinued at the time of surgery, but was resumed if IOP remained ≥ 25 mmHg after 1 month postoperative. Follow-up examinations were completed according to the surgeon’s customary postoperative schedule (at days 1 and 7; months 1, 3, and 6; and annually through 10 years).
Data Analyses
Effectiveness and safety parameters were documented for all available eyes at each visit (observed cohort analysis). Mean observed IOP and number of medications are reported. Since this was a real-world study of the surgeon’s actual clinic patients, not all patients attended all postoperative visits. To account for differences in follow-up, a consistent cohort analysis was completed alongside the observed cohort analysis at every postoperative time point. For this analysis, mean IOP or medications at each postoperative time point were compared to the IOP or medications of the same set of eyes preoperatively. Due to these multiple comparisons, a Bonferroni correction was performed with the Wilcoxon signed-rank test when comparing postoperative vs. preoperative mean IOP and medications. After this multiple-comparison correction, the significance threshold changed from 0.05 to 0.007.
To compare the proportions of preoperative vs. 10-year postoperative eyes with IOP ≤ 18 and IOP ≤ 15 mmHg, and to compare the proportions of preoperative vs. 10-year postoperative eyes on two or more medications, the McNemar–Bowker test was used, with a significance threshold of p < 0.05. To compare the proportions of preoperative vs. 10-year postoperative eyes on 0, 1, 2, 3, 4, or 5 medications, the Lehmacher test for dependent variables was used, with a significance threshold of p < 0.05.
Results
Accountability and Demographics
Initially, 70 eyes of 48 patients had been identified in the data collection, of whom 22 patients received the implant binocularly and 26 patients in one eye. Of these eyes, seven eyes had to be excluded from the evaluation: in four eyes, 10-year IOP data were not available, and in three eyes, relevant preoperative data were not available. The final study dataset included 63 eyes of 45 patients who had IOP and medication data available at the preoperative and 10-year visits.
Table 1 provides the preoperative demographic and ocular parameters of the eyes in the study. Mean age was 81.6 ± 7.3 years old (range, 60–96), approximately two-thirds of patients were female, and most patients were diagnosed with POAG (68.3%) or PXG (27.0%). Mean IOP was 18.6 ± 4.4 on 1.83 ± 1.03 mean medications; over half of eyes were being treated with ≥ 2 topical medications, and only two eyes (3.2%) were medication-free. Prior to surgery, 25.4% of patients had a “light” glaucoma disease burden (IOP ≤ 18 mmHg and ≤ 1 medication); 44.4% of patients had a “medium” glaucoma disease burden (IOP > 18 mmHg OR ≥ 2 medications); and 30.2% of patients had a heavy glaucoma disease burden (IOP > 18 mmHg and ≥ 2 medications). Most cases (60/63 or 95.2%) were combined with phacoemulsification. No eyes had undergone a prior glaucoma procedure.
Efficacy
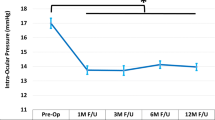
Figure 1A shows the mean IOP at each time point in all available eyes at each visit (observed cohort). Figure 1B shows the mean IOP at each postoperative time point vs. its respective preoperative value (consistent cohorts). The IOP reduction vs. preoperative ranged from 12.9 to 19.0%, and was significant at every postoperative study time point (Fig. 1B; Wilcoxon signed-rank test with Bonferroni correction; all p < 0.005). At 10 years postoperative, mean IOP decreased from 18.6 to 16.2 mmHg (12.9% reduction; p = 0.001).
A Mean intraocular pressure over time, observed cohort of all available eyes at each visit. Vertical bars represent standard deviation. B Mean intraocular pressure, consistent cohorts (each postoperative time point compared to its own respective baseline*). *Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons. Vertical bars represent standard deviation. IOP intraocular pressure
Figure 2 depicts the percentage of eyes with IOP ≤ 15 or ≤ 18 mmHg at each study time point. For both thresholds, approximately 50% more eyes reached the goal at 10 years vs. preoperative (77.8 vs. 50.8% of eyes with IOP ≤ 18 mmHg, and 47.6 vs. 25.4% of eyes with IOP ≤ 15 mmHg) (McNemar–Bowker test; p = 0.016).
Figure 3A depicts the mean number of medications at each time point in all available eyes at each visit (observed cohort). Figure 3B shows the mean number of medications at each postoperative time point vs. its respective preoperative value (consistent cohorts). The mean medication reduction vs. preoperative ranged from 37.8 to 51.4%, and was significant at every postoperative study time point (Fig. 3B; Wilcoxon signed rank test with Bonferroni correction; all p ≤ 0.006). At 10 years postoperative, mean number of medications reduced from 1.83 to 0.89 medications (51.4% reduction; p < 0.001).
A Mean number of medications over time, observed cohort of all available eyes at each visit. Vertical bars represent standard deviation. B Mean number of medications, consistent cohorts (each postoperative time point compared to its own respective baseline*). *Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons. Vertical bars represent standard deviation
Figure 4A depicts the proportions of eyes on different numbers of glaucoma medications at the preoperative vs. 10-year postoperative visit. The proportion of eyes on two or more active agents decreased from 55.6% (35 eyes) preoperatively to 17.5% (11 eyes) at the 10-year follow-up (McNemar–Bowker test; p = 0.005); meanwhile, the proportion of medication-free eyes rose from 3.2% (two eyes) preoperatively to 33.3% (21 eyes) at 10 years (Lehmacher test for dependent variables; p < 0.001). In comparison to their preoperative regimen, 93.7% of eyes either maintained or decreased their medication burden at 10 years postoperative (Fig. 4B).
Safety
All patients underwent implantation of a single iStent device; three cases were standalone procedures and 60 cases were preceded by standard phacoemulsification cataract surgery. No intraoperative or perioperative complications occurred. Over 10 years of follow-up, nine eyes had nine secondary glaucoma surgeries: one deep sclerectomy (at 1 month postoperative), three cyclophotocoagulation procedures (at 1, 3, and 5 years postoperative), and four additional iStent implantations (two eyes at 1 year and two eyes at 6 years postoperative). No filtering surgeries such as trabeculectomy or tube shunt were completed. No sight-threatening or device-related adverse events were reported, including no hypotony maculopathy, endophthalmitis, retinal detachment, or choroidal detachment or hemorrhage.
CDVA improved after surgery, as could be expected given that 60/63 of eyes had cataract surgery alongside iStent implantation. Improvement in mean CDVA was maintained through 10 years postoperative. Over the 10-year follow-up, five eyes developed new or worsening age-related macular degeneration and non-glaucomatous optic atrophy. As a result, we did two separate analyses of mean visual acuity: without and with these eyes. The analysis without these eyes is presented in Fig. 5a and b. The analysis with these eyes is presented in Fig. 6a and b.
A Proportional analysis of corrected-distance visual acuity preoperatively and at 5 and 10 years postoperatively, eyes without age-related macular degeneration and optic atrophy at the 10-year follow-up. B Mean corrected-distance visual acuity over follow-up, eyes without age-related macular degeneration and optic atrophy at the 10-year follow-up. Vertical bars represent standard deviation
A Proportional analysis of corrected-distance visual acuity preoperatively and at 5 and 10 years postoperatively, including eyes with age-related macular degeneration and optic atrophy at the 10-year follow-up. B Mean corrected-distance visual acuity over follow-up, including eyes with age-related macular degeneration and optic atrophy at the 10-year follow-up. Vertical bars represent standard deviation
Discussion
This paper presents some of the longest-term data to date for any glaucoma procedure, including both MIGS and traditional filtering surgery. To our knowledge, this is the first 10-year publication for any MIGS device. The paper evaluates longitudinal effectiveness and safety of iStent implantation with phacoemulsification in a real-world clinical population. Clinically and statistically, significant reductions in IOP and medication burden were achieved, accompanied by favorable safety outcomes including preserved visual acuity and no filtering surgery over 10 years.
The importance of reducing IOP and medication burden cannot be overstated. Lowering IOP is widely accepted to be the cornerstone of glaucoma treatment. Landmark glaucoma trials have established the importance of any degree of IOP lowering in the prevention of glaucoma development or progression. This includes the Early Manifest Glaucoma Trial, which showed a 10% lower risk of glaucoma progression for every 1 mmHg reduction in IOP [12, 13]; the Ocular Hypertension Treatment Study, which showed approximately 10% lower risk of developing glaucoma for every 1 mmHg of IOP reduction [14]; and the Canadian Glaucoma Study, which showed a 19% lower risk of glaucoma progression for every 1 mmHg of IOP reduction [15]. These findings underline the value of even modest IOP reductions.
Alongside IOP reduction, the mean medication burden in our cohort decreased by 37.8–51.4%, with significantly greater proportions of medication-free eyes at 10 years vs. preoperative. Of note, nearly all eyes maintained or reduced medication burden at 10 years vs. preoperative. There are many worthwhile benefits of reducing medication burden. Medications are associated with well-documented deleterious effects to the ocular surface and periorbital tissues [16], IOP fluctuations [17, 18], higher rates of subsequent glaucoma surgical failure [19], lower quality of life [20, 21], costs [22], difficulty with administration [23], and high rates of nonadherence and nonpersistence [24, 25]. Noncompliance can lead to greater visual decline in patients with glaucoma [26]. Even when used with perfect compliance, topical medications are prone to more IOP fluctuations than a surgical intervention [27, 28]; these IOP fluctuations, in turn, predispose to greater glaucomatous damage [29, 30]. It is no wonder, then, that studies have shown surgical interventions to preserve vision better than topical medications [31]. In addition, earlier surgical intervention has been shown to impact the trajectory of the disease, contributing to a delay or avoidance of visual decline in patients with glaucoma [32].
Two prior publications, with 8-year and 7-year results, respectively, may be particularly informative to contextualize the current 10-year outcomes. Salimi et al. studied the 8-year effectiveness and safety of implanting two first-generation iStent trabecular micro-bypass stents alongside cataract surgery [8]. They found that mean IOP reduced by 26% (from 19.2 mmHg preoperatively to 14.2 mmHg at 8 years, p < 0.001) and mean medications decreased by 17.9% (from 2.8 to 2.3, p = 0.018). At 8 years postoperative, 91.1% of eyes achieved IOP ≤ 18 mmHg (vs. 51.6% preoperatively), and 69.6% of eyes achieved IOP ≤ 15 mmHg (vs. 14.5% preoperatively). In comparison to the Salimi paper, our cohort had smaller IOP reductions (12.8–18.9%) and greater medication reductions (37.8–51.4%). These IOP differences could be due to the implantation of two stents (Salimi et al.) vs. one stent (our cohort), as multiple stents are known to have greater effect than single stents [33]. The medication differences could be attributed to the higher preoperative medication burden in the Salimi cohort, which may imply a more advanced disease state with a greater difficulty in reducing medication burden postoperatively. Both studies had favorable safety, including low (Salimi et al.) or no (our cohort) filtering surgery during follow-up.
Hengerer et al. examined 7-year outcomes of iStent inject implantation either with or without cataract surgery (Combined and Standalone subgroups, respectively) [34]. They reported 34.1–38.9% IOP reduction and 57.9–69.0% medication reduction in the Combined subgroup over the 7 years of follow-up (p < 0.001 throughout). In comparison to the Hengerer paper, our cohort had smaller postoperative IOP reductions. This could be attributed to the implantation of two vs. one stent (similar to the comparison between our study and Salimi et al., above). It also could be due to the higher preoperative IOP in the Hengerer paper, which is known to produce greater percent reduction postoperatively [35]. Meanwhile, the percentage medication reduction was similar in the two studies. Both studies had similar rates of secondary surgeries, and no filtering surgeries.
Limitations of the Study
The present study has several limitations given its retrospective, unmasked, nonrandomized design. As this was a real-world patient population in the surgeon’s own clinic, washouts were not indicated nor would they have been appropriate. However, the absence of washouts makes the postoperative IOP reductions particularly noteworthy, as nearly all patients were using topical medications preoperatively. All patients in whom cataract surgery was planned and who were using topical anti-glaucomatous therapy were offered simultaneous iStent implantation in order to reduce or eliminate topical treatment burden. As with all non-randomized studies of glaucoma surgery with concomitant phacoemulsification, the effect of cataract surgery could not be separated from that of the glaucoma procedure. However, post-phacoemulsification IOP reduction in treated patients with glaucoma is typically modest: less than 2 mmHg on average [35,36,37,38], or a reduction versus baseline of 16.5% 3 years after cataract extraction, as shown in the Ocular Hypertension Treatment Study (OHTS) [35]. In addition, evidence has shown that the reduction in IOP may be more significant at 1 year after cataract surgery, and that subsequently IOP tends to return to baseline levels with time [38,39,40,41]. Thus, any IOP decrease from cataract surgery in this study would be expected to dissipate by the 10-year follow-up. Not all subjects were available at every study visit; however, we employed consistent cohort analyses to enable statistical comparisons even while accounting for differing numbers of patients at each postoperative follow-up. Visual field examinations were not available over the 10-year timespan. However, visual acuity was gathered as a surrogate marker of visual function, and these results were reassuringly stable.
Conclusions
Despite these limitations, the present study provides some of the longest-term published data on any MIGS intervention. It does so in a realistic clinical setting with heterogeneous patients including those with open-angle glaucoma or ocular hypertension, differing numbers of preoperative topical medications, and differing visual acuities. The results showed sustained long-term reduction in IOP and medications, together with favorable safety including preserved visual acuity, no intraoperative complications, and no filtration surgeries over the 10-year follow-up.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Susanna R Jr, et al. Why do people (Still) go blind from glaucoma? Transl Vis Sci Technol. 2015;4(2):1.
Malihi M, et al. Long-term trends in glaucoma-related blindness in Olmsted County. Minnesota Ophthalmology. 2014;121(1):134–41.
Radcliffe NM, Shah M, Samuelson TW. Challenging the “Topical Medications-First” approach to glaucoma: a treatment paradigm in evolution. Ophthalmol Ther. 2023;12(6):2823–39.
Bedrood S, et al. Alternatives to topical glaucoma medication for glaucoma management. Clin Ophthalmol. 2023;17:3899–913.
Gazzard G, et al. Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess. 2019;23(31):1–102.
Yang SA, et al. Trends and usage patterns of minimally invasive glaucoma surgery in the United States: IRIS(R) registry analysis 2013–2018. Ophthalmol Glaucoma. 2021;4(6):558–68.
Ma AK, et al. GlaucoMap—distribution of glaucoma surgical procedures in the United States. Clin Ophthalmol. 2020;14:2551–60.
Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis (Lond). 2021;8(1):43.
Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41(12):2664–71.
Neuhann TH, et al. Long-term effectiveness and safety of trabecular microbypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension: five-year outcomes. J Cataract Refract Surg. 2019;45(3):312–20.
Samuelson TW, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–67.
Heijl A, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–79.
Leske MC, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56.
Gordon MO, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–20 (discussion 829-30).
Chauhan BC, et al. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–6.
Kolko M, et al. Impact of glaucoma medications on the ocular surface and how ocular surface disease can influence glaucoma treatment. Ocul Surf. 2023;29:456–68.
Stewart WC, et al. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117–22 (e1).
Walters TR, et al. 24-Hour IOP control with once-daily bimatoprost, timolol gel-forming solution, or latanoprost: a 1-month, randomized, comparative clinical trial. Surv Ophthalmol. 2004;49(Suppl 1):S26-35.
Broadway DC, et al. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–54.
Nordmann JP, et al. Vision related quality of life and topical glaucoma treatment side effects. Health Qual Life Outcomes. 2003;1:75.
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9 (e2).
Patel AR, et al. Economic and clinical burden associated with intensification of glaucoma topical therapy: a US claims-based analysis. J Glaucoma. 2021;30(3):242–50.
Lanzl IM, Poimenidou M, Spaeth GL. Possibilities and limitations of eye drops for glaucoma therapy. Ophthalmologe. 2016;113(10):824–32.
Nordstrom BL, et al. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606.
Olthoff CM, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–61.
Newman-Casey PA, et al. The association between medication adherence and visual field progression in the collaborative initial glaucoma treatment study. Ophthalmology. 2020;127(4):477–83.
Konstas AG, et al. 24-hour intraocular pressure control with maximum medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology. 2006;113(5):761–5 (e1).
Muniesa M, Ezpeleta J, Benitez I. Fluctuations of the intraocular pressure in medically versus surgically treated glaucoma patients by a contact lens sensor. Am J Ophthalmol. 2019;207:429–30.
Nouri-Mahdavi K, et al. Predictive factors for glaucomatous visual field progression in the advanced glaucoma intervention study. Ophthalmology. 2004;111(9):1627–35.
Asrani S, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9(2):134–42.
Musch DC, et al. Intraocular pressure control and long-term visual field loss in the collaborative initial glaucoma treatment study. Ophthalmology. 2011;118(9):1766–73.
Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol. 2008;145(2):191–2.
Katz LJ, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–62.
Hengerer FH, Auffarth GU, Conrad-Hengerer I. 7-Year efficacy and safety of iStent inject trabecular micro-bypass in combined and standalone usage. Adv Ther. 2024;41:1481–95.
Mansberger SL, et al. Reduction in intraocular pressure after cataract extraction: the ocular hypertension treatment study. Ophthalmology. 2012;119(9):1826–31.
Friedman DS, et al. Surgical strategies for coexisting glaucoma and cataract: an evidence-based update. Ophthalmology. 2002;109(10):1902–13.
Vizzeri G, Weinreb RN. Cataract surgery and glaucoma. Curr Opin Ophthalmol. 2010;21(1):20–4.
Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34(5):735–42.
Mathalone N, et al. Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. J Cataract Refract Surg. 2005;31(3):479–83.
Shingleton BJ, et al. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–90.
Kim DD, Doyle JW, Smith MF. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg Lasers. 1999;30(1):37–40.
Acknowledgements
The authors wish to thank the patients for their participation in this study.
Medical Writing/Editorial Assistance
Independent third-party data analysis was provided by Monika Fuchs and Katja Zoric of TargoMed. This data analysis was funded by Glaukos Corporation.
Funding
There was no financial support for the work in this study. The publication fee was funded by Glaukos Corporation.
Author information
Authors and Affiliations
Contributions
Tobias H. Neuhann: study concept and design; data collection; drafting; revising; decision to submit. Raphael T. Neuhann: study concept and design; data collection; drafting; revising; decision to submit. Dana M. Hornbeak: study concept and design; data analysis; literature review; drafting; revising; decision to submit.
Corresponding author
Ethics declarations
Conflict of Interest
Tobias H. Neuhann and Raphael T. Neuhann have nothing to disclose. Dana M. Hornbeak is an employee and shareholder of Glaukos Corporation.
Ethical Approval
All visits were conducted according to the tenets of the Declaration of Helsinki and the ethical standards of the responsible committee on human research (Bayrische Landesärztekammer München); informed consent was completed for all patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Neuhann, T.H., Neuhann, R.T. & Hornbeak, D.M. Ten-Year Effectiveness and Safety of Trabecular Micro-Bypass Stent Implantation with Cataract Surgery in Patients with Glaucoma or Ocular Hypertension. Ophthalmol Ther 13, 2243–2254 (2024). https://doi.org/10.1007/s40123-024-00984-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00984-1