Abstract
Introduction
The aim of this work was to compare the prognosis and characteristics of patients with Cytomegalovirus (CMV) infection (CMV+) with those of patients without virus infection (Virus–) undergoing repeat keratoplasty.
Methods
This prospective propensity score-matched cohort study enrolled patients who underwent repeat keratoplasty for graft failure at the Peking University Third Hospital between January 2016 and May 2022. Patients with prior viral keratitis before the first keratoplasty were excluded. The primary outcome measure was the graft failure rate. The secondary outcome measures included the anterior segment characteristics, intraocular pressure (IOP), and endothelial cell density.
Results
Ninety-four matched patient pairs were included. The graft failure rate in the CMV+ group (71%) was higher than that in the Virus– group (29%) (P < 0.001). CMV infection in the cornea increased the risk of repeat graft failure and shortened the median survival time (hazard ratio, 3.876; 95% confidence intervals, 2.554–5.884; P < 0.001). The characteristics of graft failure included exacerbation of ocular surface inflammation, neovascularization, and opacification. Epithelial defects, high IOP, and endothelial decompensation were observed at an increased frequency in the CMV+ group (all P < 0.005). Recurrent CMV infection presented as early endothelial infection in the CMV+ group. Recurrence of CMV infection was confined to the graft endothelium without involving the stroma and epithelium post-repeat endothelial keratoplasty.
Conclusions
CMV infection post-keratoplasty leads to persistent endothelial damage and graft opacification and significantly increases the risk of repeat graft failure. Localized recurrence of CMV infection in the endothelial grafts underscores the importance of monitoring and treatment.
Trial Registration
Chictr.org.cn, ChiCTR1800014684.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Cytomegalovirus (CMV) infection is a known cause of corneal graft failure leading to repeat penetrating keratoplasty. |
Data detailing the graft failure rates and clinical features of patients with CMV infection undergoing repeat keratoplasty remain insufficient. |
What was learned from this study? |
CMV infection post-keratoplasty leads to persistent endothelial damage and graft opacification and significantly increases the risk of repeat graft failure. |
CMV infection following previous keratoplasty significantly escalates the risk of repeat graft failure, which underscores the critical need for re-enhanced and targeted CMV prevention and treatment strategies in such high-risk scenarios. |
Introduction
Cytomegalovirus (CMV), a herpesvirus, is prevalent globally, with an estimated CMV immunoglobulin G (IgG) seroprevalence of 66–90% [1]. CMV persists in latent form following initial infection in humans and reactivates episodically to replicate and shed virions, leading to clinical manifestations [2]. CMV is observed more frequently than other herpes viruses in recipients of organ transplants, particularly kidney transplants [3, 4], and is linked to postoperative complications and graft failure.
Post-keratoplasty CMV corneal infection remains a significant complication [5], elevating graft failure rate to > 70% [6]. CMV infection causes corneal graft failure which leads to repeated penetrating keratoplasty (PKP) [7, 8]; however, data detailing graft failure rates and clinical features of patients with CMV infection undergoing repeat keratoplasty remain insufficient.
CMV damages corneal tissue, particularly the endothelium, through direct viral replication and immune-mediated inflammation [5]. Early suspicion aids in the diagnosis and timely implementation of antiviral therapy [5]. Coin-shaped lesions and “owl-eye cells” under confocal microscopy are characteristic signs of CMV endothelial infection [9]. Other signs include focal corneal edema and linear keratic precipitates (KPs) [10]. However, advanced graft edema and opacification complicate CMV identification. Viral deoxyribonucleic acid (DNA) polymerase chain reaction is the most sensitive diagnostic tool post-keratoplasty [6]. However, repeat paracentesis is necessary to diagnose CMV infection with viral DNA polymerase chain reaction, so clear clinical indicators are needed to initiate detection and mitigate repeated paracentesis-associated risks.
This study investigated CMV corneal infection's impact on repeat keratoplasty's prognostic outcomes and clinical features. We compared outcomes and clinical characteristics in CMV-positive (CMV+) and virus-negative (Virus–) patients who underwent repeat keratoplasty.
Methods
Study Participants
This prospective cohort study adhered to the Declaration of Helsinki, and was approved by the Peking University Third Hospital Ethics Committee (No. M2017314; M2021283) and registered with the Chinese Clinical Trial Registry (ChiCTR1800014684). Informed consent was obtained from all participants.
Patients who underwent repeat keratoplasty owing to graft failure at Peking University Third Hospital between January 2016 and May 2022 were classified into the CMV+ (exposed; detected with > 100 copies/ml CMV DNA in DNA extraction solution from aqueous humor or cornea tissue and without other virus DNA) or Virus– (unexposed; detected with no herpes virus DNA in DNA extraction solution from aqueous humor or cornea tissue) groups at a 1:2 ratio based on the viral DNA results of the corneal tissue or aqueous humor collected during repeat keratoplasty. Follow-up concluded on September 30, 2022. Loss to follow-up was defined as absence of clinical visits 6 months post-operatively.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) history of previous graft failure and (2) detection of herpes virus DNA during repeat keratoplasty. Exclusion criteria were: (1) history of viral keratitis before the previous transplantation, (2) missing data, (3) suspected viral keratitis with negative DNA detection results, and (4) infection with other types of herpes viruses.
Primary Processing of Baseline Data
Supplementary Material presents the baseline data collected from the CMV+ and Virus– patients. Q-type clustering was performed on CMV+ patients according to the baseline data for subgroup analysis, then propensity score matching (PSM) was performed to balance the two groups at a ratio of 1:1, based on clustering.
Measurements and Outcomes
The anterior segment characteristics were evaluated 1, 3, 6, and 12 months postoperatively and annually thereafter. Aqueous humor samples of patients were used for viral DNA detection whenever CMV infection was suspected. The primary outcome measure was the failure rate, characterized by corneal opacification (Supplementary Material), irreversible edema, and regrafting necessity [11]. The evaluation of corneal grafts in our study adheres to the criteria established by Holland et al. [12]. Secondary clinical outcomes included anterior segment features post-repeat keratoplasty (opacification, ocular surface vessels, edema, epithelial defect, neovascularization, infection, and immune inflammatory ulcer), intraocular pressure (IOP; Tx-F, Canon Inc, Japan), endothelial cell density (ECD) and loss (HRT III, Heidelberg Engineering), central corneal thickness (CCT; Anterior-segment OCT, Carl Zeiss Meditec), and viral DNA detection.
Keratoplasty and Viral DNA Detection
All keratoplasty procedures, including PKP, automated lamellar keratoplasty (ALK), and endothelial keratoplasty (EK), were performed by the same surgeon (J.H.). Corneal tissue and aqueous humor were sampled for virus detection (herpes simplex virus-1[HSV-1], HSV-2, CMV, varicella-zoster virus [VZV], Epstein–Barr virus [EBV]) during repeat keratoplasty. Additional aqueous humor samples were collected for the same purpose whenever CMV infection was suspected before and after surgery. The Supplementary Material describes the keratoplasty techniques and viral DNA detection methods.
Drug Treatment
Standard care post-repeat keratoplasty included 0.5% levofloxacin (Cravit) topically for 1 month and 1.0% prednisolone (Pred Forte) acetate four times daily, gradually reduced over 3–6 months. Additionally, topical 1.0% cyclosporine or 0.1% tacrolimus (Protopic) was initiated 1 week post-surgery and tapered according to graft status.
Antiviral therapy (intravenous ganciclovir [5 mg/kg; Li Kele, China Meheco Keyi Pharma Co.] for 14–28 days, followed by oral ganciclovir [1 g thrice daily; Li Kewei, China Meheco Keyi Pharma Co.] and 0.15% ganciclovir eye cream [Li Keming, China Meheco Keyi Pharma Co.] six times daily until surgery) was commenced immediately for patients with CMV+ before repeat keratoplasty. The CMV+ group received intravenous ganciclovir for 28 days postoperatively and oral ganciclovir and ganciclovir eye cream four times daily for a minimum of 6–12 months. Blood cell counts were monitored. Poor responders received intravitreal ganciclovir injection (3 mg/0.1 ml) [13] or second-line foscarnet sodium and sodium chloride (Kenai, China Zhengda Tianqing Pharma Co., China) empiric therapy (3 g/250 ml intravenous twice daily) [14].
Sample Size
Sample-size calculations were performed using PASS (version 15.0; NCSS LLC., Kaysville, UT, USA), incorporating nine variables in a logistic regression model for Q-type clustering analysis. The required sample size was estimated at 135 cases, considering 15 events per variable. The target sample size was set at ~ 85 patients in both the exposed and unexposed groups, factoring a 20% data loss rate.
Data Analysis
Statistical analysis utilized SPSS (IBM Corp., Armonk, NY), R Studio v4.2.2 (Boston, MA), and Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). A two-tailed P value of < 0.05 was considered statistically significant. Missing data were addressed using the last observation carried forward method. Normally and non-normally distributed data are presented as mean ± standard deviation (SD) and median with interquartile range, respectively. Categorical data are presented as numbers (percentages). Survival curves for post-repeat keratoplasty were generated using Kaplan–Meier and landmark analyses, with log-rank tests comparing failure rates, hazard ratios (HR), and 95% confidence intervals (CI) between groups. Independent t tests and chi-square tests were employed to analyze continuous and dichotomous variables, respectively. A sensitivity analysis was conducted for unmatched data.
Results
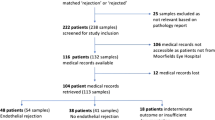
Of 423 patients, 45 (11%) were excluded owing to loss to follow-up (n = 40) and primary graft failure (n = 5). Thus, 126 CMV+ and 252 Virus– patients were included. Figure 1 illustrates the patient flow. Initial analysis revealed baseline imbalances between the groups (Tables S1–3 in Supplementary Material). Cluster analysis of the CMV+ patients yielded four distinct groups: post-PKP/ALK (n = 40), post-EK (n = 12), local/systemic dysimmunity-related keratopathy (immune dysfunction; n = 38), and congenital corneal opacification (CCO; n = 36) groups (Fig. 2 and Supplemental Methods).
Baseline Data in Unmatched and Matched Groups
PSM included five covariates: age, congenital corneal opacification, dysimmunity-related keratopathy, and keratoplasty methods (PKP/ALK and EK). Ninety-four pairs of patients were matched across four subgroups within the CMV+ and Virus– groups. Table 1 presents the baseline characteristics. Post-matching, the SD for all covariates was reduced to < 0.1. The data between groups reached a good balance after matching. The scatter plot of SD before and after match is presented in Figure S1 (Supplementary Material). Median follow-up duration after matching was 6.5 (3–11.3) and 8.5 (4–15.3) months in the CMV+ and Virus– groups, respectively.
Primary Outcome Indicator
Prognostic Outcome in the Matched Group
Repeat graft failure occurred in 93 patients (50%) in the matched group at the end of follow-up: 71% (66/93) were CMV+ with a failure density of 9% (66/742) person-months, and 29% (27/93) were Virus– with a failure density of 3% (27/1096) person-months. The repeat keratoplasty failure rate was significantly higher in the CMV+ group (log-rank test, P < 0.001). CMV corneal infection emerged as a crucial risk factor for repeat keratoplasty (HR, 3.876; 95% CI, 2.554–5.884; P < 0.001; Fig. 3A). The median graft survival duration in the CMV+ group was shorter (10 months; 95% CI, 0.5–26) than that in the Virus– group (27 months; 95% CI, 3–52). The 6–12-month period post-repeat keratoplasty was identified as crucial for CMV-related graft failure (landmark analysis, P < 0.05; Fig. 3B and C).
Secondary Outcome Indicator
Clinical Characteristics of Repeat Failed Corneal Grafts
Repeat graft failures in the CMV+ group were marked by stromal neovascularization, ocular surface vasodilation, and severe opacification (all P < 0.001) compared with the Virus– group (Fig. 4C). CMV infection heightened the risk of complications (high IOP and epithelial defects) (P < 0.001; Fig. 4B and C).
Clinical characteristics of the patients with repeat failed corneal grafts. *P < 0.05; **P < 0.01; ***P < 0.001. A Comparison of the clinical characteristics of the CMV+ and Virus– patients with repeat failed corneal grafts. B Clinical characteristics of the CMV+ patients with repeat failed corneal grafts. Severe opacification (a–f), stromal neovascularization (a, d, f), corneal perforation, stromal neovascularization, ocular surface vasodilation (g), corneal epithelial defect (f–i). C Clinical characteristics of repeat failed corneal grafts in Virus– patients. Simple edema without ocular surface vasodilation can be observed in the cornea, even with confined corneal dissolution (c). CMV Cytomegalovirus, IOP intraocular pressure, NV neovascularization, OS ocular surface, II immune inflammatory, CMV+ Cytomegalovirus positive, Virus– virus negative
Clinical Characteristics in CMV+ Group
Table S4 (in Supplementary Material) presents the findings of viral DNA detection in the aqueous humor of the CMV+ group post-repeat keratoplasty. In the CMV+ group, 63% (59/94) were solely CMV DNA-positive, 2% (2/94) only EBV-positive, 6% (6/94) EBV and CMV-positive, and 29% (27/94) negative for all herpesvirus DNA. CMV DNA was predominantly identified within the first 1–3 months postoperatively (Fig. 5A). Distinct clinical features (ocular surface vasodilation, corneal edema, and pigment-containing KPs) (Fig. 5B and C([e–g, k–m, a–d]) and complications (corneal epithelial defects and elevated IOP) (Fig. 5B and C[h–j]) were observed more frequently in patients with repeat graft failure than those without in the CMV+ group. Viral DNA was not detected in the Virus– group during follow-up.
Clinical characteristics of patients in the CMV+ group with recurrent CMV infection after repeat keratoplasty. *P < 0.05; **P < 0.01; ***P < 0.001. A Time of CMV DNA detection after repeat keratoplasty in months. B Comparison of the clinical characteristics of the CMV DNA positive and viral DNA negative patients in the CMV+ group. C a–d: KPs containing pigments, C e–g: Ocular surface vasodilation, C h–j: Corneal epithelial defect, C k–m: Stroma edema with inflammatory cell infiltration. CMV Cytomegalovirus, CS coin-shaped, DNA deoxyribonucleic acid, IOP intraocular pressure, KPs keratic precipitates, OS ocular surface, CMV + Cytomegalovirus positive, Virus– virus negative
ECD, ECD Loss, and CCT
Sixty and 72 patients in the CMV+ and Virus– groups, respectively, were assessed to evaluate corneal endothelial decompensation. Forty-five and 64 CMV+ and Virus– patients were evaluated to assess ECD, ECD loss, and CCT. The CMV+ group exhibited a higher incidence of endothelial decompensation post-repeat keratoplasty (P < 0.001; Fig. 6A). Monthly ECD in the CMV+ group was consistently lower than that in the Virus– group (P < 0.001). The 6–12-month period post-repeat keratoplasty was crucial for ECD loss related to CMV infection (P < 0.001; Fig. 6B). CCT was significantly thicker at 12 months post-repeat keratoplasty in the CMV+ group (P = 0.02; Fig. 6C).
Endothelial characteristics after repeat keratoplasty. *P < 0.05; **P < 0.01; ***P < 0.001. A Corneal endothelial decompensation after repeat keratoplasty. B ECD and ECD loss after repeat keratoplasty. C CCT 12 months post-repeat keratoplasty. CCT central corneal thickness, CMV Cytomegalovirus, ECD endothelial cell density, EK endothelial keratoplasty, PKP penetrating keratoplasty, CMV + Cytomegalovirus positive, Virus– virus negative
Subgroup Analysis
Primary outcomes of repeat keratoplasty in subgroups aligned with the overall group findings (Figure S2A, C, E, and G in Supplementary Material), which indicates that CMV corneal infection heightened repeat graft failure risk (P < 0.001). The period from the 12-month post-PKP/ALK to last follow-up, and the 1–6-month period post-EK were identified as pivotal periods for CMV-related graft failure (landmark analysis, both P < 0.05; Figures S1B and 1C). Antiviral therapy before repeat keratoplasty was a protective factor for graft survival (HR 0.519; 95% CI 0.309–0.873; P = 0.002) (Figure S2G in Supplementary Material) that extended the median graft survival duration, but did not significantly affect the graft failure rate (P = 0.41).
Exploratory Analysis
Effect of Keratoplasty Methods on CMV Infection
Table S5 (in the Supplementary Material) presents keratoplasty methods used in the CMV+ group. The PKP–EK sequence in the EK subgroup exhibited a higher repeat graft failure rate than that in EK–EK (PKP–EK: 75%, EK–EK: 60%). Post-PKP–EK lesions extended from the endothelium to the stroma, whereas post-EK–EK lesions were confined to the endothelium. The PKP–PKP sequence showed a failure rate (54/72) of 75% in the PKP subgroup, with lesions affecting the entire corneal stroma. However, CMV infection post-EK–EK affected only the endothelium, in contrast to the PKP–EK and PKP–PKP sequences, where the infection spread from the endothelium to the stroma, ultimately affecting the full graft thickness (Fig. 7).
CMV infection following different keratoplasty methods. A CMV infection after PKP-EK sequence. A coin-shaped lesion can be observed in the endothelial layer after repeat keratoplasty by EK (A b, red arrow); ECD loss and morphological changes; (A b); severe, full corneal thickness opacification in the failed graft (A c). B CMV infection after PKP–PKP sequence. Pigment-containing KPs can be observed in the endothelial layer after the second PKP (B, red arrow); epithelial defect with edema in the failed graft (B c). C CMV infection after EK–EK sequence. ECD loss and morphological change after repeat keratoplasty (C b-c). CCT central corneal thickness, CMV Cytomegalovirus, ECD endothelial cell density, EK endothelial keratoplasty, KPs keratic precipitates, PKP penetrating keratoplasty, CMV+ Cytomegalovirus positive
Sensitivity Analysis
The prognostic outcomes of repeat keratoplasty in 126 CMV+ and 252 Virus– patients without matching were examined. Repeat graft failure was observed in 153 patients (41%) up to the end of follow-up: 96 (76%) from the CMV+ group with a failure density of 11% (96/894) person-months, and 57 (23%) from the Virus– group with a failure density of 3% (27/1096) person-months. CMV infection was a significant risk factor (HR, 3.375; 95% CI, 2.432–4.684; P < 0.001). The median graft survival duration was notably shorter in the CMV+ group (8 months; 95% CI, 6–10) than that in the Virus– group (27 months; 95% CI, 20–34). The 6–12-month period post-repeat keratoplasty was crucial for CMV-related graft failure (landmark analysis, P < 0.05; Figure S3 in Supplementary Material). These results confirm the robustness of the main findings.
Discussion
This study highlights the detrimental impact of CMV corneal infection on repeat keratoplasty, demonstrating a significantly high graft failure rate of 70%. Recurrent CMV infection presents with notable clinical features and complications, including ocular surface vasodilation, pigment-containing KPs, epithelial defects, elevated IOP, and endothelial decompensation. These findings underscore the critical requirement for focused management strategies for patients with CMV infection undergoing keratoplasty, considering its substantial role in graft failure.
CMV Exerted Persistent Destructive Effects on the Corneal Allograft Following Repeat Keratoplasty
The failure rate for low-risk keratoplasty ranges from 0 to 20% [15, 16]. However, this rate increases to 50% for grafts with pre-operative corneal inflammation and vascularization [17]. In our annual 500–600 keratoplasties, the failure rate of primary grafts is approximately 5% per year. This study focused on repeat keratoplasties with varying degrees of corneal vascularization and revealed that these are not low-risk procedures. The failure rate escalated to 70% in the presence of CMV infection, highlighting its profound impact on corneal grafts.
An optimal antiviral treatment has not been established for CMV corneal infections [9]. Ganciclovir and valganciclovir remain the first-line drugs, complemented by various mono and combination therapies, such as topical, intravitreal, intravenous, or oral administration [9, 13]. Valganciclovir possesses superior oral bioavailability [18]; however, its high cost limits its use in China. In this study, long-term intravenous ganciclovir was selected to ensure maximum bioavailability [19] and safety [9]. Persistent antiviral therapy post-keratoplasty prolongs the graft survival duration; however, it does not reduce the failure rates substantially, indicating the requirement for better CMV management strategies, particularly for high-risk patients with post-keratoplasty CMV reactivation.
Clinical Characteristics of Recurrent CMV Infection Post-Repeat Keratoplasty
Recurrent CMV infection, which presents as CMV endotheliitis, typically occurs within 1–3 months post-repeat keratoplasty and is characterized by ocular surface vasodilation, corneal edema, and presence of pigment-containing KPs. Ocular surface vasodilation is present consistently from the initial stage of recurrent infection to graft failure in CMV+ patients, but exhibits varying patterns among different subgroups. The dilated vessel branches occasionally formed flower ring-like patterns around the corneal margin or graft-host interface in the PKP/ALK subgroup (Fig. 5C e), potentially connecting with ciliary vessels or extending into the deeper corneal layers (Fig. 5C g). One or two dilated vessels extended towards the corneal margin but seldom reached the endothelial graft in the EK subgroup, without connecting to the ciliary vessels (Fig. 5C f). Minimal ocular surface vasodilation was observed in the patients in the Virus– group with repeat graft failure.
This study identified pigment-containing KPs, observed in various forms at the edges of linear endothelial lesions, as a hallmark sign of CMV endotheliitis post-repeat keratoplasty. This pigment distribution predominantly occurs during the early stages of CMV infection, and diminishes, giving way to other clinical manifestations affecting anterior chamber and corneal stroma as CMV progresses to these areas. This transition underlines the dynamic nature of the impact of CMV infection on the cornea.
CMV can infect various tissues in the anterior segment, including the corneal endothelium, iris, and trabecular meshwork, and lead to endothelial decompensation and high IOP [20]. High IOP, reported in 10–36% of patients, is a common complication post-keratoplasty and can be caused by the administration of steroids, collapse of the trabecular meshwork due to surgical procedures, and iris-corneal adhesion [21, 22]. Our study also revealed a notable association between CMV corneal infection and increased IOP post-repeat keratoplasty, a correlation that had been under-recognized previously. This increase in IOP was especially pronounced in early stage CMV infection within the CCO subgroup. Recent studies suggest a link between CCO and congenital CMV infection [23]. The exact mechanism remains to be fully elucidated, and corneal transplantation could potentially exacerbate these symptoms.
CMV presents a significant risk to corneal endothelial cells, causing damage directly through viral replication and indirectly via immune-mediated inflammation [24]. This dual assault disrupts endothelial cell structure, leading to endothelial decompensation [25]. Our findings demonstrate that CMV markedly influences ECD and its loss post-repeat keratoplasty, leading to a notable association between CCT and CMV infection 12 months post-surgery. This timeframe aligns with that reported by Marcus, indicating that endothelial cell loss occurs for approximately 10 months post-keratoplasty [26], which is consistent with the time point in our study.
Consistent with the findings of studies that identify ocular HSV infection as a risk factor for epithelial defects post-keratoplasty [21], this study indicates a similar risk posed by CMV corneal infection. Tan et al. reported a case of epithelial defect after Descemet membrane EK, which was suspected to be related to CMV infection after excluding other possible causes [5]. The incidence of epithelial defects was higher in the immune dysfunction subgroup, indicating the pivotal role of CMV in inducing ocular surface complications [27]. Initial epithelial defects [28] and subsequent epithelial healing dysfunction are caused by CMV and lead to ocular surface complications [29]. Similar to alveolar cells [30] and retinal neural epithelial cells [31], corneal epithelial cells are likely to be CMV targets. CMV DNA detection in the epithelial layer of grafts further substantiates the direct impact of CMV on the corneal epithelium.
This study established a link between deep corneal neovascularization and CMV corneal stroma infection. Corneal vascularization heightens the risk of immune response, thereby increasing the likelihood of graft failure [17, 32]. CMV infection exacerbates this risk by promoting neovascularization, particularly in the PKP/ALK subgroup. However, the effects of CMV were confined to the endothelium, and less neovascularization was observed in the EK subgroup. This difference may be attributed to the layer of CMV infection, indicating that its progression beyond the endothelium to the stroma is a critical factor influencing neovascularization and subsequent graft failure [5].
Our study underscores that CMV significantly damages corneal allografts, contrasting to systemic CMV infections observed in other organ transplants [33]. The corneal stroma may be a CMV latency reservoir [10]. CMV can transfer from the corneal stroma of the host to the graft endothelium after repeat full-thickness transplanting, promoting virus replication and progression to the stroma and anterior chamber. CMV infection after EK–EK procedures largely affects the endothelial grafts, sparing the stroma and epithelium. Moreover, CMV-associated graft failure often follows rapid endothelial cell loss, highlighting the impact of CMV endotheliitis on graft viability [26]. These patterns indicate a strong connection between CMV infection and transplant rejection, thus emphasizing the need to monitor and treat CMV infection. This study included only two cases each of ALK-PKP and ALK-ALK sequences in the CMV+ group. Our data also showed that CMV infection after keratoplasty often occurs following PKP or ALK while the incidence of which following EK is relatively low (Supplementary Material). Therefore, the number of EK group in our cohort was relatively small. Thus, further research is warranted to better understand the role of CMV in different transplant scenarios.
Limitations of the study are as follows: a single-center study conducted at a tertiary referral center may result in referral selection bias; amplified by a wide follow-up range (6–120 months, median 12 months). Absence of standardized antiviral treatment regimens may have introduced confusion in the subgroup analysis. Considering the high prevalence of CMV IgG positivity reported in China [34], the inability to detect CMV infection in donor corneas represents a limitation. This may introduce confounding factors into the rate of CMV infection following repeat keratoplasty. Lastly, the study lacks an analysis of the reasons for failure of primary grafts, as although CMV infection contributes to increased failure rate of repeat grafts, discerning its direct effects from those mediated by rejection remains challenging [35].
Conclusions
CMV infection following previous keratoplasty significantly escalates the risk of repeat graft failure. Recurrent infection manifests as early endothelial infection with typical anterior segment findings. Failed grafts with CMV show severe endothelial decompensation and corneal opacification. These findings underscore the critical need for re-enhanced and targeted CMV prevention and treatment strategies in such high-risk scenarios. CMV recurrence tends to be localized to the graft endothelium post-repeat EK, sparing the recipient’s cornea to a greater extent.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of Cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034. https://doi.org/10.1002/rmv.2034. ([published Online First: Epub Date]).
Haidar G, Boeckh M, Singh N. Cytomegalovirus infection in solid organ and hematopoietic cell transplantation: state of the evidence. J Infect Dis. 2020;221(Suppl 1):S23-s31. https://doi.org/10.1093/infdis/jiz454. ([published Online First: Epub Date]).
Einsele H, Ljungman P, Boeckh M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. 2020;135(19):1619–29. https://doi.org/10.1182/blood.2019000956. ([published Online First: Epub Date]).
Mohiuddin MM, Singh AK, Scobie L, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet. 2023;402(10399):397–410. https://doi.org/10.1016/s0140-6736(23)00775-4. ([published Online First: Epub Date]).
Tan TE, Tan DTH. Cytomegalovirus corneal endotheliitis after Descemet membrane endothelial keratoplasty. Cornea. 2019;38(4):413–8. https://doi.org/10.1097/ico.0000000000001847. ([published Online First: Epub Date]).
Hsiao CH, Hwang YS, Chuang WY, et al. Prevalence and clinical consequences of Cytomegalovirus DNA in the aqueous humour and corneal transplants. Br J Ophthalmol. 2018. https://doi.org/10.1136/bjophthalmol-2018-312196. ([published Online First: Epub Date]).
Shahrudin NA, Mohd Zahidin AZ, Md Noh UK, Wan Abdul Halim WH, Md DN. CMV endotheliitis: a cause for recurrent failed corneal transplant. GMS Ophthalmol Cases. 2017;7:31. https://doi.org/10.3205/oc000082. ([published Online First: Epub Date]).
Fernández López E, Chan E. Descemet stripping automated endothelial keratoplasty outcomes in patients with Cytomegalovirus endotheliitis. Cornea. 2017;36(1):108–12. https://doi.org/10.1097/ico.0000000000001028. ([published Online First: Epub Date]).
La Distia NR, Putera I, Mayasari YD, et al. Clinical characteristics and treatment outcomes of Cytomegalovirus anterior uveitis and endotheliitis: a systematic review and meta-analysis. Surv Ophthalmol. 2022;67(4):1014–30. https://doi.org/10.1016/j.survophthal.2021.12.006. ([published Online First: Epub Date]).
Chan AS, Mehta JS, Al Jajeh I, Iqbal J, Anshu A, Tan DT. Histological features of Cytomegalovirus-related corneal graft infections, its associated features and clinical significance. Br J Ophthalmol. 2016;100(5):601–6. https://doi.org/10.1136/bjophthalmol-2015-307390. ([published Online First: Epub Date]).
Terry MA, Aldave AJ, Szczotka-Flynn LB, et al. Donor, recipient, and operative factors associated with graft success in the cornea preservation time study. Ophthalmology. 2018;125(11):1700–9. https://doi.org/10.1016/j.ophtha.2018.08.002. ([published Online First: Epub Date]).
Holland EJ, Chan CC, Wetzig RP, Palestine AG, Nussenblatt RB. Clinical and immunohistologic studies of corneal rejection in the rat penetrating keratoplasty model. Cornea. 1991;10(5):374–80. https://doi.org/10.1097/00003226-199109000-00003. ([published Online First: Epub Date]).
Yu T, Peng RM, Xiao GG, Feng LN, Hong J. Clinical evaluation of intravitreal injection of ganciclovir in refractory corneal endotheliitis. Ocul Immunol Inflamm. 2020;28(2):270–80. https://doi.org/10.1080/09273948.2019.1573261. ([published Online First: Epub Date]).
Meesing A, Razonable RR. New developments in the management of Cytomegalovirus infection after transplantation. Drugs. 2018;78(11):1085–103. https://doi.org/10.1007/s40265-018-0943-1. ([published Online First: Epub Date]).
Pavlovic I, Shajari M, Herrmann E, Schmack I, Lencova A, Kohnen T. Meta-analysis of postoperative outcome parameters comparing Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Cornea. 2017;36(12):1445–51. https://doi.org/10.1097/ico.0000000000001384. ([published Online First: Epub Date]).
Birbal RS, Ni Dhubhghaill S, Bourgonje VJA, et al. Five-year graft survival and clinical outcomes of 500 consecutive cases after Descemet membrane endothelial keratoplasty. Cornea. 2020;39(3):290–7. https://doi.org/10.1097/ico.0000000000002120. ([published Online First: Epub Date]).
Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117(7):1300-5.e7. https://doi.org/10.1016/j.ophtha.2010.01.039. ([published Online First: Epub Date]).
Cheng YC, Kang EY, Hwang YS, Hsiao CH. Treatment of Cytomegalovirus anterior segment infection with intravitreal injection of ganciclovir in adjunction with or without oral valganciclovir: a long-term results. Sci Rep. 2021;11(1):3105. https://doi.org/10.1038/s41598-021-82637-y. ([published Online First: Epub Date]).
Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512. https://doi.org/10.1111/ctr.13512. ([published Online First: Epub Date]).
Zhang S, Zang Y, Lu Q, et al. Establishing an animal model of Cytomegalovirus keratouveitis in rats: broad infection of anterior segment tissue by Cytomegalovirus. Invest Ophthalmol Vis Sci. 2021;62(13):22. https://doi.org/10.1167/iovs.62.13.22. ([published Online First: Epub Date]).
Alio JL, Montesel A, El Sayyad F, Barraquer RI, Arnalich-Montiel F, Alio Del Barrio JL. Corneal graft failure: an update. Br J Ophthalmol. 2021;105(8):1049–58. https://doi.org/10.1136/bjophthalmol-2020-316705. ([published Online First: Epub Date]).
Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2018;125(2):295–310. https://doi.org/10.1016/j.ophtha.2017.08.015. ([published Online First: Epub Date]).
Bateman CM, Kesson A, Powys M, Wong M, Blyth E. Cytomegalovirus infections in children with primary and secondary immune deficiencies. Viruses. 2021. https://doi.org/10.3390/v13102001. ([published Online First: Epub Date]).
Kobayashi R, Hashida N, Maruyama K, Nishida K. Clinical findings of specular microscopy images in Cytomegalovirus corneal endotheliitis. Asia Pac J Ophthalmol (Phila). 2022;11(3):273–8. https://doi.org/10.1097/apo.0000000000000522. ([published Online First: Epub Date]).
Moshirfar M, Murri MS, Shah TJ, et al. A review of corneal endotheliitis and endotheliopathy: differential diagnosis, evaluation, and treatment. Ophthalmol Ther. 2019;8(2):195–213. https://doi.org/10.1007/s40123-019-0169-7. ([published Online First: Epub Date]).
Ang M, Sng CC, Chee SP, Tan DT, Mehta JS. Outcomes of corneal transplantation for irreversible corneal decompensation secondary to corneal endotheliitis in Asian eyes. Am J Ophthalmol. 2013;156(2):260-66.e2. https://doi.org/10.1016/j.ajo.2013.03.020. ([published Online First: Epub Date]).
Choi SH, Kim MK, Oh JY. Corneal limbal stem cell deficiency in children with Stevens–Johnson syndrome. Am J Ophthalmol. 2019;199:1–8. https://doi.org/10.1016/j.ajo.2018.10.016. ([published Online First: Epub Date]).
Choy BNK, Ng ALK, Zhu MM, Liu CC, Xu S, Lai JSM. Randomized control trial on the effectiveness of collagen cross-linking on bullous keratopathy. Cornea. 2020;39(11):1341–7. https://doi.org/10.1097/ico.0000000000002395. ([published Online First: Epub Date]).
Li L, Li Y, Zhu X, et al. Conjunctiva resident γδ T cells expressed high level of IL-17A and promoted the severity of dry eye. Invest Ophthalmol Vis Sci. 2022;63(12):13. https://doi.org/10.1167/iovs.63.12.13. ([published Online First: Epub Date]).
Yunis J, Farrell HE, Bruce K, et al. Murine Cytomegalovirus glycoprotein O promotes epithelial cell infection in vivo. J Virol. 2019. https://doi.org/10.1128/jvi.01378-18. ([published Online First: Epub Date]).
Schampera MS, Schweinzer K, Abele H, et al. Comparison of Cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: Impact of CMV IgG normalization. J Clin Virol. 2017;90:40–5. https://doi.org/10.1016/j.jcv.2017.03.005. ([published Online First: Epub Date]).
Nicholas MP, Mysore N. Corneal neovascularization. Exp Eye Res. 2021;202:108363. https://doi.org/10.1016/j.exer.2020.108363. ([published Online First: Epub Date]).
Wang TZ, Kodiyanplakkal RPL, Calfee DP. Antimicrobial resistance in nephrology. Nat Rev Nephrol. 2019;15(8):463–81. https://doi.org/10.1038/s41581-019-0150-7. ([published Online First: Epub Date]).
Chen J, Hu L, Wu M, Zhong T, Zhou YH, Hu Y. Kinetics of IgG antibody to Cytomegalovirus (CMV) after birth and seroprevalence of anti-CMV IgG in Chinese children. Virol J. 2012;9:304. https://doi.org/10.1186/1743-422x-9-304. ([published Online First: Epub Date]).
Li Y, Yan H, Xue WJ, et al. Allograft rejection-related gene expression in the endothelial cells of renal transplantation recipients after Cytomegalovirus infection. J Zhejiang Univ Sci B. 2009;10(11):820–8. https://doi.org/10.1631/jzus.B0920115. ([published Online First: Epub Date]).
Acknowledgements
We thank all study participants for their involvement.
Funding
The study was funded by the National Natural Science Foundation. The grant number is 82371027. The foundation had no role in the design or conduct of this study. The journal’s Rapid Service Fee was funded by Peking University Third Hospital. None of the authors has any financial or proprietary interests in any material or methods mentioned.
Author information
Authors and Affiliations
Contributions
Yunxiao Zang, Rongmei Peng, and Jing Hong contributed to the study conception and design. Yaning Zhao, Yi Qu, Xuanjun Zhang, and Jiaxin Zhang contributed to data collection and analysis. The first draft of the manuscript was written by Yunxiao Zang and Yaning Zhao. Xiaozhen Liu, Gege Xiao, and Jing Hong commented on previous versions of the manuscript. Jing Hong read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Yaning Zhao, Rongmei Peng, Gege Xiao, Xiaozhen Liu, Yi Qu, Xuanjun Zhang, Jiaxin Zhang, and Jing Hong do not have any conflicts of interest to disclose. Yunxiao Zang changed the affiliation after the completion of the manuscript. The current affiliation of Yunxiao Zang is Beijing Tongren Hospital, Capital Medical University and Beijing Ophthalmology.
Ethical Approval
This prospective cohort study adhered to the Declaration of Helsinki, and was approved by the Peking University Third Hospital Ethics Committee (No. M2017314; M2021283) and registered with the Chinese Clinical Trial Registry (ChiCTR1800014684). Informed consent was obtained from all participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zang, Y., Zhao, Y., Peng, R. et al. Incidence of Cytomegalovirus Infection After Repeat Keratoplasty and Associated Rate of Graft Failure. Ophthalmol Ther 13, 1967–1980 (2024). https://doi.org/10.1007/s40123-024-00968-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00968-1