Abstract
Introduction
The aim of this work is to compare 20 intraocular lens (IOL) power calculation formulas in medium-long eyes (24.50–25.99 mm) in terms of root mean square absolute error (RMSAE), median absolute error (MedAE), and percentage of eyes with prediction error (PE) within ± 0.50 D.
Methods
The data of patients who underwent uneventful phacoemulsification between January 2017 and September 2023 were reviewed. Pre-surgery IOL power was calculated using Holladay1, SRK/T, Hoffer Q, Holladay 2, and Haigis. Three months after phacoemulsification, refraction was measured. Post-surgery IOL power calculations were performed utilizing the following formulas: Barrett Universal II, Kane, K6, Olsen (OLCR), Olsen (standalone), PEARL-DGS, Ladas Super Formula AI (LSF AI), T2, EVO, VRF, Hoffer QST, Castrop, VRF-G, Karmona, and Naeser 2. RMSAE, MedAE, and percentage of eyes with PE within ± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 were calculated.
Results
One hundred twenty-four eyes with axial length ranges between 24.52 and 25.97 mm were studied. The SRK/T formula yielded the lowest RMSAE (0.206) just before Holladay 1 (0.260) and T2 (0.261). In terms of MedAE, the best outcome was obtained by SRK/T (0.12) followed by Barrett Universal II (0.15) and LSF AI (0.15). The highest percentage of eyes with prediction error within ± 0.50 D was achieved by SRK/T, T2, and Holladay 1 (97.58, 93.55, and 93.55%, respectively).
Conclusions
Third-generation formulas (SRK/T, Holladay 1) provided highly accurate outcomes in medium-long eyes and still can be wildly used to calculate IOL power.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intraocular lens (IOL) power calculation is a very important aspect of cataract surgery. |
Many IOL power calculation formulas have been developed over the years. |
The accuracy of the IOL formula depends on the eyeball axial length (AL). |
There is still no agreement among cataract surgeons regarding the choice of the formula. |
Medium-long eyes (24.50 mm < AL < 25.99 mm) have so far been rarely studied in terms of accuracy of IOL power calculation formulas. |
Despite many newer methods, the third-generation formulas still yield accurate IOL power calculation outcomes in medium-long eyes. |
A significant majority of IOL power calculation formulas obtain good results in medium-long eyes. |
SRK/T, as the most easily accessible formula, can guide clinicians to obtain relatively accurate calculation results when the hardware environment is limited. |
Introduction
Sir Nicolas Harold Lloyd Ridley was the first person to successfully implant an intraocular lens (IOL) on November 29, 1949, at St Thomas’ Hospital in London. However, the patient obtained refractive error of − 18.0 D sphere and − 6.0 D cylinder [1]. Cornelius Dingeman Binkhorst of the Netherlands had similar problems with achieving proper refraction after cataract surgery. Thus, it was realized that in addition to choosing the right artificial IOL material, it is necessary to accurately calculate its power. This is one of the most important factors affecting the refractive outcome of phacoemulsification [2].
Svyatoslav Nikolaevich Fyodorov, a Russian ophthalmologist, launched the first wildly known IOL power calculation formula in 1967 [3], and although many other formulas have been developed since then, the accuracy of the implant power calculation is still debatable. According to the European Registry of Quality Outcomes for Cataract and Refractive Surgery, the percentage of eyes with prediction error (PE) within ± 0.5D after cataract surgery is 73.7% [4]. However, patients’ expectations are much higher.
Currently, we have many IOL power calculation formulas. There are various classifications but the most practical one is based on a logical approach [5], as shown in Table 1. Most IOL power calculations are accurate for eyes with axial length (AL) in the range of 22.0 to 24.5 mm. However, their accuracy for eyes shorter than 22.0 mm and longer than 24.5 mm is still questionable [6, 7]. Many articles have been published on this topic, especially for long eyes [8,9,10,11,12,13,14,15].
Myopic eyeballs can vary considerably in length, which can exceed even 30 mm. In turn, the lower limit of AL often varies. Most often it is 26.0 mm [16,17,18], although the American Academy of Ophthalmology defines high myopia as eyes with a measured AL of at least 26 mm [19]. Sometimes this lower limit is 25.5 mm [20, 21], often 25.0 mm [22, 23], less often 24.5 mm [24], or even 24.0 mm [25]. Due to the above discrepancies, some authors have called this disputed range of AL between 24.5 and 25.99 mm medium-long eyes [26,27,28], and although the accuracy of the IOL power calculation formulas has sometimes been considered additionally in this AL range, it has never been the subject of a separate article.
Many different tools can be employed to assess the accuracy of the chosen formula. Most research in this area is based on observing the mean absolute error (MAE) and the percentage of eyes with PE within ± 0.50 D, less often of the median absolute error (MedAE) [16,17,18, 20, 24, 26, 27]. Hoffer et al. [29] recommended to utilize MedAE as a primary outcome in this type of studies due to the not-normal distribution of absolute refractive PE. In turn, Cooke et al. [30] proposed mean rank score for subgroup analysis (e.g., in terms of AL). Recently, most studies are based on outcomes of the root mean square absolute error (RMSAE) [31,32,33]. Then RMSAE is compared between formulas using the bootstrap-t method with Holm sequential correction [32]. It is an alternative to SD for describing the distribution of PEs for subgroups with non-zero predicted errors, such as short, long, medium-long, or eyes after corneal refractive surgery [32, 34].
This study aimed to compare the IOL power calculation formulas for medium-long eyes, i.e., with AL ranging between 24.50 and 25.99 mm in terms of RMSAE, MedAE, and the percentage of eyes with PE within ± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 D. To the best of our knowledge, this is the first study on this AL interval with so many patients. It is pioneering due to its idea. Until now, medium-long eyes have only been considered as part of studies of the entire AL range [26, 28]. Additionally, a list of as many as 20 most often used formulas proves the reliability of the study.
Methods
The data of patients with eyes of AL ranging from 24.50 to 25.99 mm and with Wisconsin grade 3 or 4 cataract who underwent uneventful sutureless phacoemulsification with monofocal IOL implantation with a 2.4-mm clear corneal incision between January 2018 and September 2023 were retrospectively reviewed. Rigorous exclusion criteria were applied such as corneal astigmatism greater than 2.0 D, postoperative BCVA less than 0.8, a history of other ophthalmic procedures, i.e., vitrectomy, limbal relaxing incisions, and corneal refractive surgery, any intraoperative or postoperative complications as well as previous corneal diseases.
The study was conducted adhering to the tenets of the Declaration of Helsinki. Each patient signed informed consent for routine cataract surgery. The study was approved by the Institutional Review Board of Foundation for the Advancement of Ophthalmology “Ophthalmology 21” (1/2024).
Preoperative optical biometry was performed using a Zeiss IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) obtaining the following data for each patient: AL, keratometry (K), anterior chamber depth (ACD), lens thickness (LT), and white to white (WTW) as corneal diameter. Central corneal thickness was taken from auto kerato-refracto tonometer TRK-2P (Topcon Corporation, Tokyo, Japan). Prior to cataract surgery, the IOL power was calculated using Holladay 1, SRK/T, Hoffer Q, Holladay 2, Haigis. In patients operated on in 2020, IOL power was additionally calculated using the Barrett Universal II formula. The power of the implanted IOL was randomly selected from the outcomes of one of the following formulas, i.e., SRK/T, Holladay 2 or Barrett Universal II. Each cataract surgery was performed by the same eye surgeon. Acrylic foldable intraocular lenses were implanted (AcrySof IQ SN60WF – Alcon Laboratories, Fort Worth, TX, USA). Postoperative refraction was measured 3 months after cataract surgery. Post-surgery IOL power was calculated utilizing the following formulas: Barrett Universal II, Ladas Super Formula AI (LSF AI), Kane, EVO 2.0, Pearl-DGS, T2, VRF, VRF-G, Olsen (OLCR), Olsen (standalone), K6, Castrop, Hoffer QST, Karmona and Naeser 2. We used optimized lens constants for the AcrySof IQ SN60WF IOL from the ULIB website, and did not use optimization to achieve zero mean predicted error. This lens is well known and studied, and these optimized constants were derived from a big data group of eyes (> 5000 eyes).
The IOLMaster 700 (software version 1.7, Carl Zeiss, Meditec AG) was used for IOL power calculation by Holladay 1, SRK/T, Hoffer Q, Holladay 2 and Haigis formulas. Optimized constants from the ULIB for the IQ SN60WF IOL were used for these formulas. Calculations using Castrop, Naeser 2, T2, VRF, and VRF-G formulas were performed by one of the authors (OV).
The respective web resources were used to calculate Hoffer QST (https://hofferqst.com/), LSF AI (https://www.iolcalc.com/) and Karmona (https://karmona-iol.com/) formulas. Calculations and data analysis for EVO 2.0, K6, Kane, and Pearl-DGS were performed for us by authors of these formulas via personal communications (Yeo TK, Cooke DL, Kane J, Debellemanière G). Additionally, David L Cooke performed calculations related to Barrett Universal II, Olsen (OLCR), and Olsen (standalone) formulas. The optimized A-constant of 119.00 or its respective values was used for all methods. The keratometric index was used of 1.3375.
PE was defined as the difference between the actual postoperative refractive outcome expressed as spherical equivalent (equal to the sum of the spherical power and half the cylindrical power) and the refraction predicted by each formula. A positive value indicated a hyperopic error and a negative value referred to myopic error while absolute value was absolute error (AE). Based on the AE percentage of patients with a correction of ± 0.25 D, ± 0.50 D, ± 0.75 D, ± 1.00 D was obtained.
Statistical Analysis
Data were analyzed in IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA) and R Project 4.3.0 for Statistical Computing (https://www.r-project.org/). The normality of PE distribution was assessed with the Kolmogorov–Smirnov test. The RMSAE and mean of PE were chosen as primary outcomes. The RMSAE was compared between formulas using the bootstrap-t method with Holm sequential correction as an alternative to SD for describing the distribution of PEs for subgroups with non-zero predicted errors. Additionally, MedAE was considered, also. A p value less than 0.05 was considered statistically significant. A nonparametric Cochran Q test with the McNemar post hoc test were used to compare the percentage of eyes with a PE within ± 0.50 D. The Bonferroni correction was applied for the post hoc tests. A minimum sample size of 94 eyes was needed to have a confidence level of 95% that the real value is within ± 5% of the measured value (PS program, Version 3.0.12; Dupont WD, 2012).
Results
Ninety-five patients (39 men and 56 women) were included in the study. One hundred twenty-four eyes (51 male eyes and 73 female eyes) were examined. The AL of the studied eyes ranged from 24.52 to 25.97 mm. Demographic details of enrolled patients and biometric data of comprised eyes are listed in Table 2.
PE of most formulas was slightly negative, which was consistent with the general trend of IOL power calculation. However, five formulas yielded slightly positive PE (Holladay 2, Karmona, PEARL-DGS, SRK/T and VRF). Interestingly, PEARL DGS achieved a PE closest to zero (0.001).
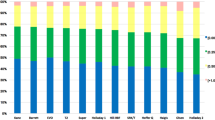
Out of the 20 evaluated formulas, the SRK/T obtained the lowest RMSAE (0.206) followed by Holladay 1 (0.260), T2 (0.261), LSF AI (0.270) and Barrett Universal II (0.289). In turn, Olsen (OLCR), Castrop and Naeser 2 yielded the highest RMSAE (0.504, 0.442, and 0.400, respectively). Detailed results of the RMSAE for each formula were summarized in Fig. 1. Statistical comparison of the RMSAE was performed using the Bootstrap-t method with the Holm correction. Statistically significant differences were found for the SRK/T (P < 0.000), Holladay 1 (P < 0.000), T2 (P < 0.000), Castrop (P < 0.000), Barrett (P < 0.000), LSF AI (P < 0.000), Olsen (standalone) (P < 0.000) and Olsen (OLCR) (P < 0.000). Detailed outcomes are shown in Table 3.
In terms of the MedAE, the best results were obtained by SRK/T (0.120), Barrett Universal II (0.150), and LSF AI (0.150), and the worst by Olsen (OLCR) (0.315), Castrop (0.261), and Holladay 2 (0.255), as shown in Fig. 2.
The highest percentage of patients with PE within ± 0.50 D was found for SRK/T (97.58%) followed by T2 (93.55%), Holladay 1 (93.55%), LSF AI (92.74%), and VRF-G (91.94%). Olsen (OLCR), Castrop, and Naeser 2 yielded the smallest percentage of patients within PE ± 0.50 D (71.77, 75.00, and 79.03%, respectively), as shown in Fig. 3. Multiple comparisons of the formulas according to percentage of eyes with PE within ≤ 0.50 D according to the Cochran Q test with the McNemar post hoc paired test are summarized in Table 4. The second column shows each formula for which differences were statistically significant with the other formulas (P < 0.05).
Results of optimized constants and all refractive outcomes, i.e., PE, RMSAE, SD, mean absolute deviation (MAD), MedAE, MAE, as well as percentage of patients within PE ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D for all studied formulas are summarized in Table 5.
Discussion
This study demonstrated that the SRK/T formula achieved the lowest RMSAE (0.206) and the highest percentage of patients within PE ± 0.50 D (97.58%). Although at first glance the result seems surprising, after all, the SRK/T formula was considered universal for many years and is still widely used in many biometric devices e.g., Zeiss IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany). Recent studies still show very high accuracy of SRK/T. Priji et al. in their 2021 study considering accuracy of six formulas (Hill-RBF, Kane, Barrett Universal, Hoffer Q, Holladay1, and SRK/T) demonstrated that the SRK/T formula had the lowest MAE in eyes with AL exceeding 24.0 mm (0.11). It is interesting to note that Holladay 1 produced second outcome (0.17) ahead of Hill-RBF, Barrett Universal and Kane [25]. This is consistent with our results.
The SRK/T formula was published by John Retzlaff, Donald Sanders, and Manus Kraff in 1990. SRK named after formula developers and T meant theoretical. The formula was developed using the nonlinear terms of the theoretical formulas as its foundation but empirical regression methodology for optimization. Postoperative ACD prediction, retinal thickness axial length correction, and corneal refractive index were systematically and interactively optimized using an iterative process on five data sets consisting of 1677 posterior chamber lens cases [35].
Kenneth Hoffer was one of the first to consider medium-long eyeballs when evaluating the accuracy of 4 IOL formulas (Holladay 2, Holladay 1, Hoffer Q, and SRK/T) [36]. He included 317 eyes in his study, 52 of which were medium-long (AL ranged from 24.5 to 26.00 mm). SRK/T obtained the lowest MAE (0.345) followed by Holladay 1, Hoffer Q, and Holladay 2 (0.368, 0.465, and 0.505). Therefore, the order is the same as in our study. However, his results are much worse because to measure AL of the eyeball Hoffer used the immersion ultrasound technique, which is much less accurate than IOL-Master method applying swept optical coherence tomography [37].
A similar study but on a larger group of eyes (n = 643) was conducted a few years later by Narváez et al. Medium-long group (24.5–26.0 mm) included 317 eyes. The authors concluded that there was no significant difference in the accuracy of the four aforementioned formulas in terms of MAE. SRK/T and Holladay 1 obtained equal outcomes (0.49). However, lack of Haigis and Olsen formulas is a strong limitation of that study. Immersion ultrasound technique used to biometry is another its limitation [38].
A very robust study based on 8108 eyes was published by Aristodemou et al. They realized that for ALs from 24.5 to 25.99 mm there was a trend toward lower MAEs for Holladay 1, with statistical differences in both IOL subgroups i.e., Sopfort AO and Akreos Fit (Bausch & Lomb, Rochester, NY, USA). In turn, in terms of percentage of eyes with a PE within ± 0.50 D SRK/T obtained the highest outcome for AL from 25.5 to 25.99 mm and Holladay 1 for AL from 24.5 to 25.49 mm. Importantly, IOLMaster device was used for biometry measurement in all cases. However, Haigis, Olsen, T2, and Barrett formulas were not considered, which is a limitation of their study [39].
Kane et al. compared accuracy of the third- and fourth-generation formulas with newer (Hill-RBF, Ladas, FullMonte) in their 2017 study. They comprised 3122 eyes of which 340 were medium-long. Among all ten studied formulas, considering entire AL range SRK/T yielded second lowest MAE and MedAE (0.40, 0.33, respectively) just after Barrett Universal II (0.38, 0.30, respectively). In turn, in medium-log eyes Holladay 1 achieved second lowest MAE (0.37) recognizing superiority of Barrett (0.33) [26]. However, they utilized first version of the Hill-RBF formula, which was less accurate than currently used based on large data set (over 30,000 eyes) Hill-RBF 3.0 [40, 41].
Another study whose results are consistent with ours was published in 2018 [28]. The author assessed trueness of seven IOL power calculation formulas (Holladay 1, Hoffer Q, SRK/T, Holladay 2, Haigis, T2, and VRF) in 494 eyes including 70 medium-long (AL ≥ 24.5 mm to < 26.0 mm). He realized that Holladay 1 obtained the lowest MAE (0.346) followed by T2 (0.348). Considering the percentage of eyes with PE within ± 0.50 D the best outcome was achieved also by Holladay 1 (74.3%) just before SRK/T (72.8%). However, the lack of Olsen, Barrett, Kane, and PEARL-DGS is a significant limitation of this study.
Contrarily, Voytsekhivskyy et al. in their 2023 study involving 300 patients showed that in medium-long eyes (AL from 24.5 to 25.99 mm) the Hill-RBF 3.0 formula yielded the lowest MedAE followed by VRF-G and Kane (0.156, 0.161, and 0.176, respectively). They tested as many as 24 formulas of which PEARL-DGS obtained the highest percentage of eyes with PE within ± 0.50D (94.83%) just before K6, Okulix and Kane (all equally resulting 94.74% each). What is interesting, Holladay 1 achieved 92.98% what was one of the best results. However, they used hydrophobic IOL Tecnis 1 ZCB00 (Johnson & Johnson Vision, New Brunswick, NJ, USA) so outcomes may vary slightly. Additionally, the small sample size of medium-long eyes (n = 58) did not allow them to perform a full-fledged statistical analysis in this subgroup, so the results were reported with informational purposes only [42].
Considering MedAE as a tool to assess the accuracy of IOL power calculation formulas, which is in line with Hoffer’s recommendations [29], we obtained the best results still for SRK/T, however Barrett was right behind it. The high accuracy of the Barrett Universal II formula is indisputable as it has been proven in many modern studies [7, 9, 11, 12, 15, 20].
There are several limitations to the study. All patients were implanted the same model of IOL, so these results may not be generalizable to IOL models of a different design. The IOLs evaluated in the study were of anterior asymmetric biconvex and many other IOL designs such as equi-biconvex were also common. The differences in IOL shape could affect PE and change the relative performance of the formula tested. Having only one eye surgeon is the next limitation of this study. Research with only a single surgeon is unlikely to reach the number of cases required for significance and may be biased. However, there are two philosophies regarding the most appropriate method for evaluating the results of IOL power formulas used in clinical practice [36]. We chose the method based on standardizing all the variables in biometric data collection by using only one surgeon and one IOL style, leaving the formulas as the only variable.
Lack of the Hill-RBF formula is next limitation of this study. The Hill-RBF formula is a pure data-driven IOL calculation approach. It employs pattern recognition developed by MATLAB and a sophisticated form of data interpolation. The formula uses a large dataset (more than 12,000 eyes in version 2.0 and over 30,000 eyes in version 3.0) [41]. Additionally, several other recent AI-based formulas (Nallasamy, Zhu-Lu, Zeiss AI) and ray-tracing (Okulix) were also skipped.
Against Hoffer et al. recommendations, both eyes of 29 patients were included [43]. However, this should not affect the results because the study included 95 patients with only 1 eye tested and a minimum sample size of 94 eyes was needed to have a confidence level of 95%.
Additionally, pupil dilatation was not considered in the study. There are studies on the influence of pupil dilation on the accuracy of IOL power calculation formulas. However, these studies concerned only a few formulas, i.e., SRK/T, Haigis, and Barrett Universal II [44].
Age of the patient was also not considered. There are reports that current IOL power calculation formulas may have variable accuracy for different age groups [45].
Finally, parameters like K, ACD, and LT were not considered in terms of the accuracy of IOL power calculation formulas in the study. On the other hand, some authors found notable biases in the prediction errors of most of the formulas when plotted versus not only AL but also K, ACD, and LT [20].
Conclusions
The study shows that SRK/T has provided highly accurate outcomes in medium-long eyes. The formula is readily available because it is on the equipment of many biometric measuring devices so still can be wildly used to calculate intraocular lens power. In clinical practice, SRK/T, as the most accessible formula, can guide clinicians to obtain relatively accurate calculation results when the hardware environment is limited, and has a certain clinical application value.
Overall, all 20 tested formulas achieved in over 70% of eyes PE within ± 0.50 D, which is in line with the European Registry of Quality Outcomes [4].
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Williams HP. Sir Harold Ridley’s vision. Br J Ophthalmol. 2001;85(9):1022–3. https://doi.org/10.1136/bjo.85.9.1022.
Stopyra W. Analysis of accuracy of twelve intraocular lens power calculation formulas for eyes with axial hyperopia. Saudi J Ophthalmol. 2023;37(2):125–30. https://doi.org/10.4103/sjopt.sjopt_64_22.
Fedorov SN, Kolinko AI. A method of calculating the optical power of the intraocular lens. Vestn Oftalmol. 1967;80(4):27–31 (Article in Russian).
Nemeth G, Modis L Jr. Accuracy of the Hill-radial basis function method and the Barrett Universal II formula. Eur J Ophthalmol. 2021;31(2):566–71. https://doi.org/10.1177/1120672120902952.
Stopyra W, Langenbucher A, Grzybowski A. Intraocular lens power calculation formulas—a systematic review. Ophthalmol Ther. 2023;12(6):2881–902. https://doi.org/10.1007/s40123-023-00799-6.
Wang Q, Jiang W, Lin T, Wu X, Lin H, Chen W. Meta-analysis of accuracy of intraocular lens power calculation formulas in short eyes. Clin Exp Ophthalmol. 2018;46(4):356–63. https://doi.org/10.1111/ceo.13058.
Wang Q, Jiang W, Lin T, Zhu Y, Chen C, Lin H. Accuracy of intraocular lens power calculation formulas in long eyes: a systemic review and meta-analysis. Clin Exp Ophthalmol. 2018;46:738–49. https://doi.org/10.1111/ceo.13184.
Ma Y, Xiong R, Liu Z, Young CA, Wu Y, Zheng D, Zhang X, Jin G. Network meta-analysis of intraocular lens power calculation formula accuracy in 1016 eyes with long axial length. Am J Ophthalmol. 2024;257:178–86. https://doi.org/10.1016/j.ajo.2023.09.009.
Stopyra W. Analysis of accuracy of twelve intraocular lens power calculation formulas for eyes with axial myopia. Taiwan J Ophthalmol. 2022;13(2):225–30. https://doi.org/10.4103/2211-5056.357849.
Voytsekhivskyy O, Tutchenko L. Comparison of prediction accuracy of 13 formulas in long eyes. Graefes Arch Clin Exp Ophthalmol. 2023;261(9):2575–83. https://doi.org/10.1007/s00417-023-06060-x.
Liu J, Wang L, Chai F, Han L, Qian S, Koch DD, Weikert MP. Comparison of intraocular lens power calculation formulas in Chinese eyes with axial myopia. J Cataract Refract Surg. 2019;45(6):725–31. https://doi.org/10.1016/j.jcrs.2019.01.018.
Rong X, He W, Zhu Q, Qian D, Lu Y, Zhu X. Intraocular lens power calculation fin eyes with extreme myopia: comparison of Barrett Universal II, Haigis and Olsen formulas. J Cataract Refract Surg. 2019;45(6):732–7. https://doi.org/10.1016/j.jcrs.2018.12.025.
Li C, Wang M, Feng R, Liang F, Liu X, He C, Fan S. Comparison of formula-specific factors and artificial intelligence formulas with axial length adjustments in bilateral cataract patients with long axial length. Ophthalmol Ther. 2022;11(5):1869–81. https://doi.org/10.1007/s40123-022-00551-6.
Li X, Wang X, Liao X. How to choose intraocular lens power calculation formulas in eyes with extremely long axial length? A systematic review and meta-analysis. PLoS One. 2024;19(1): e0296771. https://doi.org/10.1371/journal.pone.0296771.
Stopyra W. Comparison of the accuracy of six intraocular lens power calculation formulas for eyes of axial length exceeding 25.00 mm. J FR Ophthalmol. 2021;44(9):1332–9. https://doi.org/10.1016/jfo.2021.04.009.
Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane JX. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10930 eyes from the UK National Health Service. J Catarcact Refract Surg. 2020;46(1):2–7. https://doi.org/10.1016/j.jcrs.2019.08.014.
Connell BJ, Kane JX. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4(1): e000251. https://doi.org/10.1136/bmjophth-2018-000251.
Cooke DL, Cooke TL. A comparison of two methods to calculate axial length. J Cataract Refract Surg. 2019;45930:284–92. https://doi.org/10.1016/j.jcrs.2018.10.039.
Ang RE, Rapista AJ, Remo JT, Tan-Daclan MA, Cruz EM. Clinical outcomes and comparison of intraocular lens calculation formulas in eyes with long axial myopia. Taiwan J Ophthalmol. 2021;12(3):305–11. https://doi.org/10.4103/tjo.tjo_7_21.
Melles R, Holladay J, Chang W. The accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169–78. https://doi.org/10.1016/j.ophtha.2017.08.027.
Pereira A, Popovic M, Ahmed Y, Lloyd JC, El-Defrawy S, Gorfinkel J, Schlenker MB. A comparative analysis of 12 intraocular lens power formulas. Int Ophthalmol. 2021;41(12):4137–50. https://doi.org/10.1007/s10792-021-01966-z.
Carmona- González D, Castillo-Gόmez A, Palomino-Bautista C, Romero-Dominguez M, Gutiérez-Moreno MÁ. Comparison of the accuracy of 11 intraocular lens power calculation formulas. Eur J Ophthalmol. 2020;15:1120672120962030. https://doi.org/10.1177/1120672120962030.
Rocha-de-Lossada C, Colmenero-Reina E, Flikier D, et al. Intraocular lens power calculation formula accuracy: comparison of 12 formulas for trifocal hydrophilic intraocular lens. Eur J Ophthalmol. 2021;31(6):2981–8. https://doi.org/10.1177/1120672120980690.
Shammas HJ, Taroni L, Pellegrini M, Shammas MC, Jivrajka RV. Accuracy of newer intraocular lens power formulas in short and long eyes using sum-of-segments biometry. J Cataract Refract Surg. 2022;48(10):1113–20. https://doi.org/10.1097/j.jcrs.0000000000000958.
Priji P, Jacob SC, Kalikivayi L, Kalikivayi V. Correlating Kane formula with existing intraocular lens formulae for corneal curvatures and axial lengths. Oman J Ophthalmol. 2021;14(2):94–9. https://doi.org/10.4103/ojo.ojo_62_21.
Kane JX, Van Heerden A, Atik A, Petsoglou C. Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg. 2017;43(3):333–9. https://doi.org/10.1016/j.jcrs.2016.12.021.
Savini G, Di Maita M, Hoffer KJ, et al. Comparison of 13 formulas for IOL power calculation with measurements from partial coherence interferometry. Br J Ophthalmol. 2021;105(4):484–9. https://doi.org/10.1136/bjophthalmol-2020-316193.
Voytsekhivskyy OV. Development and clinical accuracy of a new intraocular lens power formula (VRF) compared to other formulas. Am J Ophthalmol. 2018;185:56–67. https://doi.org/10.1016/j.ajo.2017.10.020.
Hoffer KJ, Savini G. Update on intraocular lens power calculation study protocols: the better way to design and report clinical trials. Ophthalmology. 2021;128(11):e115–20. https://doi.org/10.1016/j.ophtha.2020.07.05.
Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42:1490–500. https://doi.org/10.1016/j.jcrs.2016.06.029.
Holladay JT, Wilcox RR, Koch DD, Wang L. Review and recommendations for univariate statistical analysis of spherical equivalent prediction error for IOL power calculations. J Cataract Refract Surg. 2021;47(1):65–77. https://doi.org/10.1097/j.jcrs.0000000000000370.
Holladay JT, Wilcox RR, Koch DD, Wang L. Statistics of prediction error for dependent and independent datasets. J Cataract Refract Surg. 2023;49(4):440–2. https://doi.org/10.1097/j.jcrs.00000000000001165.
Mo E, Feng K, Li Q, Xu J, Cen J, Li J, Zhao Y. Efficacy of corneal curvature on the accuracy of 8 intraocular lens power calculation formulas in 302 highly myopic eyes. J Cataract Refract Surg. 2023;49(12):1195–200. https://doi.org/10.1097/j.jcrs.00000000000001303.
Holladay JT, Wilcox RR, Koch DD, Wang L. Astigmatism analysis and reporting of surgically induced astigmatism and prediction error. J Cataract Refract Surg. 2022;48970:799–812. https://doi.org/10.1097/j.jcrs.0000000000000871.
Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16(3):333–40. https://doi.org/10.1016/s0886-3350(13)80705-5.
Hoffer KJ. Clinical results using the Holladay 2 intraocular lens power formula. J Cataract Refract Surg. 2000;26(8):1233–7. https://doi.org/10.1016/s0886-3350(00)00376-x.
Landers J, Goggin M. Comparison of refractive outcomes using immersion ultrasound biometry and IOLMaster biometry. Clin Exp Ophthalmol. 2009;37(6):566–9. https://doi.org/10.1111/j.1442-9071.2009.02091.x.
Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32(12):2050–3. https://doi.org/10.1016/j.jcrs.2006.09.009.
Aristodemou P, Knox cartwright NE, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. https://doi.org/10.1016/j.jcrs.2010.07.032.
Tsessler M, Cohen S, Wang L, Koch DD, Zadok D, Abulafia A. Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using heteroscedastic statistical method. J Cataract Refract Surg. 2022;48(1):37–43. https://doi.org/10.1097/j.jcrs.0000000000000702.
Stopyra W, Cooke D, Grzybowski A. A review of intraocular lens power calculation formulas based on artificial intelligence. J Clin Med. 2024;13(2):498. https://doi.org/10.3390/jcm13020498.
Voytsekhivskyy OV, Hoffer KJ, Tutchenko L, Cooke DL, Savini G. Accuracy of 24 IOL power calculation methods. J Refract Surg. 2023;39(4):249–56. https://doi.org/10.3928/1081597X-20230131-01.
Hoffer KJ, Aramberri J, Haigis W, et al. Protocols for studies of intraocular lens formula accuracy. Am J Ophthalmol. 2015;160(3):403–5. https://doi.org/10.1016/j.ajo.2015.05.029. (el).
Teshigawara T, Meguro A, Mizuki N. Influence of pupil dilation on the Barrett Universal II (new generation), Haigis (4th generation) and SRK/T (3rd generation) intraocular lens calculation formulas: a retrospective study. BMC Ophthalmol. 2020;20(1):299. https://doi.org/10.1186/s12886-020-01571-1.
Sella R, Reitblat O, Durnford KM, Pettey JH, Olson RJ, Hahn TE, Bernhisel AA, Afshari NA. The effect of patient age on some new and older IOL power calculation formulas. Acta Ophthalmol. 2023. https://doi.org/10.1111/aos.16621. (Online ahead of print).
Acknowledgements
The authors appreciate David L. Cooke for his help with calculation of K6, Barrett Universal II, and Olsen’s formulas.
Funding
No financial support was received for this submission. No funding or sponsorship was received for the publication of this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: Wiktor Stopyra, Oleksiy Voytsekhivskyy; Methodology: Wiktor Stopyra, Oleksiy Voytsekhivskyy; Data acquisition: Wiktor Stopyra; Formal analysis and investigation: Wiktor Stopyra; Statistical analysis: Oleksiy Voytsekhivskyy; Writing—original draft preparation: Wiktor Stopyra; Writing—review and editing: Andrzej Grzybowski, Oleksiy Voytsekhivskyy, Wiktor Stopyra; Supervision: Andrzej Grzybowski, Wiktor Stopyra.
Corresponding authors
Ethics declarations
Conflict of Interest
Wiktor Stopyra declares that there are no conflicts of interest with this submission. Oleksiy Voytsekhivskyy is the inventor and sole owner of the VRF and VRF-G formulas and has a patent on the method of estimation of postoperative lens position (ELP) and the calculation of optical power and is the author and copyright holder of a computer program VRF Suite V1.3. Andrzej Grzybowski is an Editorial Board member of Ophthalmology and Therapy. Andrzej Grzybowski was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
The study was conducted adhering to the tenets of the Declaration of Helsinki. Each patient signed informed consent for routine cataract surgery. The study was approved by the Institutional Review Board of Foundation for the Advancement of Ophthalmology “Ophthalmology 21” (1/2024).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stopyra, W., Voytsekhivskyy, O. & Grzybowski, A. Accuracy of 20 Intraocular Lens Power Calculation Formulas in Medium-Long Eyes. Ophthalmol Ther 13, 1893–1907 (2024). https://doi.org/10.1007/s40123-024-00954-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-024-00954-7