Abstract
Introduction
The aim of this study was to investigate the influence of ocular biometry parameters on the predictive accuracy of 10 intraocular lens (IOL) power formulas in patients with high myopia (HM).
Methods
We analyzed 202 eyes of 202 patients. The ocular biometry was determined preoperatively using an IOLMaster 700. The associations between the biometry parameters and the prediction error (PE) 1 month postoperatively were assessed. HM was defined as an axial length exceeding 26.50 mm.
Results
In patients with HM (n = 108), the K6, Emmetropia Verifying Optical (EVO), Olsen, and Barrett Universal II (BUII) formulas had the lowest absolute PEs among the 10 formulas. The ocular biometry parameters were not associated with the PE of K6, EVO, Olsen, or BUII. A longer axial length in HM eyes was associated with myopic outcomes by Kane, Hoffer QST, and VRF and hyperopic outcomes by Holladay 2 and T2. Steeper keratometry, a deeper anterior chamber, and a thicker lens were associated with a hyperopic shift in HM eyes when using VRF, Kane, and Hoffer QST, respectively. In patients without HM (n = 94), there was no difference between the formulas in absolute PE. The significant associations between the biometry parameters and PE in patients with HM were not present in patients without HM.
Conclusions
K6, EVO, Olsen, and BUII displayed high accuracy in HM eyes and were not influenced by preoperative biometry parameters. For the remaining formulas, the preoperative keratometry, anterior chamber depth, lens thickness, and axial length were possible error sources underlying an inaccurate IOL power prediction in patients with HM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Refractive surprise is common after cataract surgery in people with high myopia. |
Ocular biometry plays an important role in intraocular lens (IOL) power prediction. |
The association between preoperative ocular biometry and postoperative prediction error has been unclear in patients with cataract and high myopia. |
What was learned from the study? |
Preoperative keratometry, anterior chamber depth, lens thickness, and axial length are possible error sources for IOL power prediction when Hoffer QST, Holladay 2, Holladay 2-NLR, Kane, T2, and VRF are used in high myopic eyes. |
K6, Emmetropia Verifying Optical, Olsen, and Barrett Universal II displayed high accuracy in high myopia and were not influenced by preoperative biometry parameters. |
Introduction
Currently, despite the wide application of optical biometry, some difficulties still exist in calculating and choosing an appropriately powered intraocular lens (IOL). Such difficulties have become more relevant with the increasing expectations of cataract surgery for achieving refractive accuracy. As shown by previous studies [1, 2], the IOL power calculation in patients with high myopia (HM) is especially challenging, as the refractive outcomes become less predictable in these patients, whose eyes are characterized by excessive elongation (axial length [AL] ≥ 26.50 mm) [3, 4].
The chance for refractive surprises in HM eyes is high for several reasons. First, traditional two-variable vergence formulas, such as SRK/T and Hoffer Q, depend on keratometry (K) and AL for effective lens position (ELP) prediction [5]. The absence of internal AL adjustments greatly affects the accuracies of these formulas in extreme ocular dimensions, such as HM cases. In addition, the calculation model used in two-variable and three-variable (Haigis) vergence formulas tends to overestimate K in long eyes [6], which further leads to unexpected ELP deepening and compromised predictive accuracy. Second, as a result of calibration of optical biometry to ultrasound immersion data [7], the ALs measured in people with high myopia may longer than they actually are. In addition, as nuclear opalescence is common in high myopic cataract [8], increased lens density in the visual axis could affect the group refractive index (GRI) and possibly give rise to inaccurate GRI-based AL measurements [9, 10]. As the precision of IOL calculation is essentially determined by the accuracy of the measurement of AL [7], inaccurate AL measurements in HM eyes are an important source of error in IOL power calculations. Third, the altered characteristics of the anterior segment in long eyes may interfere with the formulas’ assumptions of IOL power, which further results in inaccurate predictions.
Although some modern formulas have generally outperformed those of prior generations [1, 2], some of the newest formulas have yielded a prediction error (PE) beyond ± 0.50 D for more than 20% of patients with HM [11,12,13,14,15,16,17,18]. Therefore, for patients with HM, the refraction prediction performance of existing IOL power formulas remains far from perfect. Considering the important role of preoperative ocular biometry in ELP prediction and IOL power calculation [19], the biometric characteristics of HM eyes represent an important possible error source of IOL power prediction. However, for patients with HM, the influence of their biometric features on the predictive accuracy of different IOL power formulas has not been clarified thus far [20].
Therefore, we designed the present study to address two main questions: (1) What is the predictive accuracy of 10 currently popular IOL power formulas (the Barrett Universal II [BUII], Emmetropia Verifying Optical [EVO], Hoffer QST, Holladay 2, Holladay 2-NLR, Kane, K6, Olsen, T2, and VRF formulas) when calculating the IOL power for patients with HM? (2) How do certain ocular biometry parameters in patients with HM (AL, K, Q value, central corneal thickness [CCT], white-to-white [WTW], anterior chamber depth [ACD], and lens thickness [LT]) influence the predictive accuracy of the different formulas?
Methods
This study was approved by the Medical Ethics Committee of the Eye & ENT Hospital (Fudan University), Shanghai, China. Informed consent was obtained from all patients included in the study. The research protocols adhered to the tenets of the Declaration of Helsinki and its subsequent revisions.
Patients
This prospective cohort study comprised 202 cataractous eyes from 202 consecutive patients who underwent cataract surgery at the Eye and ENT Hospital, Fudan University (Shanghai, China). The inclusion criteria were (1) uneventful small-incision (≤ 2.6-mm-wide surgical wound) phacoemulsification cataract surgery with the insertion of one of the following six different IOL models: Zeiss CT Asphina 409 MP (n = 53), AMO Tecnis ZA9003 (n = 57), AMO Tecnis PCB00 (n = 23), HumanOptics MC X11 ASP (n = 25), Ophtec PC 525Y Ergomax (n = 13), and Rayner Superflex Aspheric 920H (n = 31); and (2) complete preoperative ocular biometry.
The exclusion criteria of this study included a history of contact lens wearing, corneal abnormalities, pterygium, glaucoma, uveitis, zonular weakness, lens luxation, diabetic retinopathy, invalid ocular biometry, missing postoperative refractive information, and postoperative corrected distance visual acuity (CDVA) less than 20/40. We ruled out patients who had undergone pterygium surgery [21], corneal refractive surgery [22], implantable collamer lens (ICL) implantation [23], or vitrectomy surgery [24]. These cases were excluded to avoid preexisting surgery-induced changes in ocular anatomical and optical properties. In addition, patients with pathological myopia featuring severe maculopathy were also excluded because of differences in the characteristics of their postoperative refractive success [25].
If both eyes with unequal CDVA in the same patient were eligible, the eye with better CDVA was selected. If both eyes with equal CDVA in one patient were eligible, the first eye that underwent cataract surgery was selected. After completion of consecutive enrollment and eye selection, the included eyes were divided into the HM group and the non-HM group according to their ALs [3, 4]. High myopia was defined as an AL exceeding 26.5 mm [3, 4].
Ocular Biometry Measurement
For all patients, ocular biometry was performed before surgery using an Oculus Pentacam HR (software version 1.25r15) for the Q value, a Tomey EM-3000 (software version 602-210S-2A5) for the CCT, and a Zeiss IOLMaster 700 (software version 1.88) for the AL, K, WTW, ACD, and LT. The Q value was assessed on the anterior corneal surface at the 6.0-mm-diameter aperture. Only the measurements with good quality indicators were considered qualified and used in this study.
IOL Power Calculation
The IOL power was calculated using 10 currently popular IOL power formulas: BUII, EVO, Hoffer QST, Holladay 2, Holladay 2-NLR, Kane, K6, Olsen, T2, and VRF. The EVO (available at https://www.evoiolcalculator.com/; accessed on 20 Sep 2023), Kane (available at https://www.iolformula.com/; accessed on 20 Sep 2023), K6 (available at https://cookeformula.com/Calculator; accessed on 20 Sep 2023), and Hoffer QST (available at https://hofferqst.com/; accessed on 20 Sep 2023) formulas are available on their respective websites. The T2 and VRF formulas were programmed into Excel spreadsheets [26, 27]. The BUII and Holladay 2 formulas are accessible via their respective Zeiss IOLMaster 700 software programs. The Olsen formula is available in a software package for IOL power calculation (PhacoOptics, IOL Innovations ApS, Aarhus, Denmark). We used the BUII formula as the actual formula for preoperative IOL power calculation.
Subjective manifest refraction was performed 1 month postoperatively. Then, the arithmetic PE was calculated as follows:
PE was defined as positive for patients with hyperopic refractive outcomes and negative for patients with myopic refractive outcomes.
The Kane, K6, and EVO formulas were optimized by their corresponding authors. The Hoffer QST formula was optimized on its official website (available at https://hofferqst.com/; accessed on 20 Sep 2023) using the predefined Excel spreadsheet. During optimization, all recipients (including HM and non-HM recipients) of one certain IOL model were categorized into a subset and analyzed as a whole for optimization. The lens constant was modified, and for each IOL subset, the dataset was reran until the mean PE (MPE) equaled zero [1]. The new lens constants after optimization are presented in Table S1. After optimization, we calculated the median absolute error (MedAE) and mean absolute error (MAE) for the comparisons between different formulas.
Statistical Analysis
In this study, MAE and MedAE were used to compare the predictive accuracy between different formulas [1, 2], whereas PE was used to evaluate the predictive accuracy of the individual formulas, according to the method described by Savini et al. [28] The MAEs, MedAEs, MPEs, ranges of PE, standard deviations of the error (STDEVs), root mean square errors (RMSEs), quartiles of absolute error (AE), and skewness of AE are presented in Table 2.
First, to compare the predictive accuracy among the 10 formulas, we calculated and analyzed the MAE and MedAE using repeated-measures analysis of variance (ANOVA) and Friedman’s test, respectively. The Cochran’s Q test was employed to compare the percentage of eyes within 0.5 D and 1.0 D of AE [29]. If the null hypothesis was rejected, a post hoc analysis with Bonferroni correction for multiple comparisons was performed. G*power software (version 3.1.9.7, Faul, Erdfelder, Lang, & Buchner, 2020. Available at https://www.gpower.hhu.de; accessed on 26 Oct 2023) was used for post hoc power analysis of repeated measures [30].
Second, we used a multivariable linear regression model to assess the influence of the ocular biometry parameters on the predictive accuracy of each IOL power formula, according to the method described by Savini et al. [28] The dependent variable of the model was the PE of each formula after zeroing out the MPE. The arguments of the model included sex, age, AL, K, Q value, CCT, WTW, ACD, and LT. The linear regression coefficients (β values) and standardized β values were calculated using the regression model.
Data analysis was performed using IBM SPSS Statistics, version 23.0 (SPSS Inc., Chicago, Illinois, USA). P values were calculated using two-sided tests, and the significance threshold was set at 5% through all statistical analyses. The normality of the data was examined by the Kolmogorov-Smirnov test after zeroing out the MPE. The needed sample size was determined using G*power software [29, 30]. Given an effect size of 0.25, a significance level of 5%, and a test power of 95% [22], a sample size of 20 eyes would be necessary.
Results
Study Population
Our study comprised 202 eyes of 202 patients, including 108 HM eyes (53%) and 94 non-HM eyes (47%). Table 1 shows the demographics and biometry data of all patients. The mean age of all patients was 65.2 ± 11.3 years (median, 66.0 years; range, 30–97 years), and 61.9% were female. HM eyes had a mean AL of 30.30 ± 2.23 mm (median, 30.20 mm; range, 26.53–35.42 mm), a mean K of 44.0 ± 1.7 D (median, 43.8 D; range, 40.2–47.8 D), with a median IOL power of 7.0 D (mean, 7.3 ± 5.0 D; range, − 3.0–17.5 D), whereas non-HM eyes had a mean AL of 23.97 ± 1.31 mm (median, 23.85 mm; range, 21.11–26.46 mm), a mean K of 44.0 ± 1.3 D (median, 44.0 D; range, 41.4–47.1 D), with a median IOL power of 20.5 D (mean, 20.3 ± 3.1 D; range, 13.5–27.0 D).
Comparison of Formula Performance
After the MPE was zeroed out, the PEs of the BUII, EVO, Hoffer QST, Holladay 2-NLR, Kane, K6, Olsen, and VRF formulas were normally distributed. In HM eyes, statistical analysis of the MAE with repeated-measures ANOVA showed a significant difference among all 10 formulas (P < 0.001). A significant difference was found predominantly between the formulas (EVO, K6, Olsen, and BUII) with the lowest MAEs (0.39, 0.41, 0.43, and 0.47, respectively) and the other six formulas (P < 0.05).
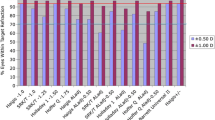
Comparison of the MedAE in HM eyes with Friedman’s test also significantly differed between all 10 formulas (P < 0.001). A significant difference was detected predominantly between the formulas (Hoffer QST, VRF, T2, and Holladay 2) with the highest MedAEs (0.45, 0.47, 0.48, and 0.61, respectively) and the remaining six formulas (P < 0.05). Figure 1 and Table 2 show the results of the 10 formulas in HM eyes. Figure 2 is a stacked histogram showing the percentages of patients within a given diopter range of the predicted refraction outcome among patients with HM. According to the results of post hoc power analysis, the power for repeated measures in the HM group was 93%.
Smoothed line graph showing the mean absolute errors and median absolute errors of the IOL power formulas in patients with high myopia. Error bars represent the 95% confidence intervals (BUII, Barrett Universal II; EVO, Emmetropia Verifying Optical; HQST, Hoffer QST; H2, Holladay 2; MAE, mean absolute error; MedAE, median absolute error; D, diopter)
Cochran’s Q test in the HM group determined that there was a statistically significant difference in the constituent ratio of AE ranges between formulas (P < 0.001). BUII, EVO, K6, and Olsen yielded significantly more AE < 0.5 D than Holladay 2 (all P < 0.05). The proportion of AE exceeding 0.5 D when utilizing T2 and VRF was significantly higher than that when using EVO and K6 (all P < 0.05). For an AE greater than 1.0 D, it was more common when Holladay 2 and T2 were employed compared with calculations by EVO, K6, and Olsen (all P < 0.05).
In patients with HM with a K < 44.0 D, the K6 (MedAE, 0.29 D), Olsen (MedAE, 0.33 D), and EVO (MedAE, 0.34 D) formulas afforded the lowest MedAEs and outperformed the Holladay 2 (MedAE, 0.59 D) formula which had a significantly higher MedAE (all P < 0.05). In HM eyes with a K ≥ 44.0 D, the K6 (MedAE, 0.31 D), EVO (MedAE, 0.34 D), Olsen (MedAE, 0.37 D), and BUII (MedAE, 0.40 D) formulas showed significantly fewer MedAEs than the Holladay 2 (MedAE, 0.62 D) formula (all P < 0.05).
In non-HM eyes, repeated-measures ANOVA with post hoc analysis showed no significant difference in the MAE among all 10 formulas (P > 0.05). Additionally, analysis of the MedAE with Friedman’s test showed no significant difference among the 10 formulas (P > 0.05). According to the results of the post hoc power analysis, the power for repeated measures in the non-HM group was 90%. Cochran’s Q test determined that in non-HM eyes, the proportion of AE exceeding 1.0 D when using Holladay 2 was significantly higher than that when K6 was employed (P < 0.05).
In non-HM eyes with a K < 44.0 D, Friedman’s test determined no significant difference among the MedAEs of the 10 formulas (all P > 0.05). However, in patients without HM with a K ≥ 44.0 D, the BUII (MedAE, 0.37 D) and K6 (MedAE, 0.35 D) formulas had significantly lower MedAEs than the Holladay 2 formula (MedAE, 0.55 D) (all P < 0.05).
Relationship Between Ocular Biometry Parameters and Prediction Error
Figure 3 is a heatmap showing the Pearson’s correlations in HM eyes between the ocular biometry parameters and the PE of each formula. For HM eyes, there was a high correlation between a greater AL and a hyperopic outcome from the Holladay 2 formula (Pearson’s correlation coefficient [r], 0.62; P < 0.001). In addition, a more negative Q value in HM eyes was correlated with hyperopic outcomes from the Holladay 2 (r, − 0.28; P = 0.007) and T2 formulas (r, − 0.28; P = 0.007). In non-HM eyes, Pearson’s correlation analysis revealed a weak correlation between the LT and the PE of the VRF formula (r, 0.24; P < 0.05).
Heatmap analysis of the correlations between the ocular biometry parameters and prediction error of each IOL power formula in patients with high myopia. Data in the cells are Pearson’s correlation coefficients (BUII, Barrett Universal II; EVO, Emmetropia Verifying Optical; HQST, Hoffer QST; H2, Holladay 2)
Table 3 summarizes the relationships of AL, K, ACD, and LT with the PEs of different formulas using the multivariable linear regression model. In HM eyes, a greater AL was associated with hyperopic refractive outcomes when using the Holladay 2 and T2 formulas (all P < 0.01) or myopic refractive outcomes when using the Kane, Hoffer QST, and VRF formulas (all P < 0.05). The formulas whose PEs had the strongest associations with AL were the Holladay 2 (β, 0.63) and Kane formulas (β, − 0.53) (Fig. 4a, b).
A hyperopic shift in HM eyes was also associated with a steeper K (for VRF, T2, and Holladay 2), greater ACD (for Kane, Holladay 2-NLR, Hoffer QST, T2, and Holladay 2), and larger LT (for Hoffer QST, Holladay 2-NLR, T2, and Holladay 2). The formulas whose PEs had the strongest association with the preoperative K, ACD, and LT were the VRF (β, 0.33), Kane (β, 0.38), and Hoffer QST formulas (β, 0.28), respectively (Fig. 4c–e). The multivariable model also revealed that WTW, CCT, and Q value had no association with the PE of any formula in the patients with HM (all P > 0.05).
According to multivariable linear regression analysis, the associations between the ocular biometry parameters and PEs that were significant in HM eyes were not present in patients without HM (all P > 0.05).
Discussion
Here, we showed that the K6, EVO, Olsen, and BUII formulas showed high accuracy in HM eyes. The accuracies of the K6, EVO, Olsen, and BUII formulas were not influenced by ocular biometry parameters. For the remaining six formulas, the preoperative K, ACD, LT, and AL were possible errors of the IOL power prediction in patients with HM.
Considering the wide variability of refractive outcomes in HM eyes [6], it is currently challenging for most ophthalmologists to choose the right IOL power formula for patients with HM, since recent studies [11,12,13,14,15,16,17,18] have demonstrated conflicting results. According to our study, we recommend the K6, EVO, Olsen, and BUII formulas for calculating the IOL power in HM eyes. The high precision of the EVO, Olsen, and BUII formulas in people with high myopia largely mirrors the findings of some recent studies [11,12,13].
According to several large cross-sectional studies [31,32,33], a greater AL was associated with lower K [31], wider WTW [32], and deeper ACD [33] in eyes with normative axial dimensions. However, the linear association between AL and K was absent when AL was greater than 28.00 mm [34]. Additionally, shallow ACD was identified in subjects with HM even with long ALs [35]. Moreover, as AL increased, the change in WTW was nonlinear, which was the same as the trend of the LT variation with ocular elongation [36]. Consideration should be given to ocular biometry alterations when calculating IOL power in high myopia.
AL is one of the most basic metrics used in IOL power formulas. Despite the constant optimization, Holladay 2 still predicts hyperopic shifts in patients with HM. In contrast, modern formulas, such as the Kane and VRF formulas, may tend to undercorrect when calculating the IOL power for long eyes to cope with the unwelcomed hyperopic shift, which is common in patients with HM [6].
It was interesting to find a relationship between the LT and the refractive outcomes of the Holladay 2 and Holladay 2-NLR formulas, as they have used the preoperative LT for calculation. According to a study by Satou et al. [37], the actual IOL position was effectively predicted by the equatorial surface depth and posterior surface depth of the crystalline lens rather than its thickness. Thus, there may be other lens biometry parameters that are more helpful than LT for predicting the ELP. According to previous findings [19], the influence of IOL position on postoperative PE was greater in normal eyes than in high-myopic eyes. A better integration of preoperative lens biometry for ELP prediction may greatly improve refractive outcomes in eyes with normative axial dimensions.
The IOLMaster 700 measures K using three different zones of the cornea. Thus, Q values are implicitly included in the K readings of the IOLMaster 700. An accurate measurement of the preoperative Q value is helpful for improving the predictive accuracy [38]. However, in our study, no relationship was found between the preoperative Q value and postoperative PE in HM eyes. According to previous large cross-sectional studies [39, 40], the Q value had a significant direct relationship with the K readings. Preoperative K is known to cause a trend error in the Haigis and SRK/T formulas in normal eyes [1, 41]. Whether the Q value was adjusted by K as a confounder in the multivariable model or whether the pattern by which the Q value and K influence IOL power in long eyes is different from that in normal eyes can only be confirmed by further studies.
Our study had several limitations. First, the optimization performed in this study is only for clinical research purposes to investigate the influence of ocular biometry on the formulas’ predictive accuracy. We did not perform an “overall optimization” of the formulas, which would be systematic work requiring a larger sample size and enough subjects from the whole AL spectrum [29]. Further investigations using lens constants from a group of normal eyes may be helpful to validate our findings. Second, more than one IOL model was used in this study, which may raise the degree of bias and affect the results of the correlation analysis. Although the MPE was zeroed separately for each IOL model, following the method described by Melles et al. [1], it would be preferable to use a single IOL model throughout the study. Third, our survey is a single-center hospital-based study with a limited sample size (total N = 202). Our study setting may enhance selection bias, which possibly gave rise to unsatisfactory normality of PE values calculated by Holladay 2 and T2. Further population-based studies with extensive sample enrollment are helpful in verifying our findings. Fourth, some new formulas, such as the Hill-RBF 3.0, PEARL-DGS, and Panacea formulas, are also attractive modern formulas deserving comprehensive investigation. However, as a result of limited space in one single paper, we confined the range of the present study to an evaluation of 10 formulas. Other new formulas, which were not included in this study, will be studied in our further survey.
Conclusions
The K6, EVO, Olsen, and BUII formulas achieved satisfactory results in HM eyes and were not influenced by preoperative ocular biometry parameters. The preoperative K, ACD, LT, and AL were possible error sources for IOL power prediction when using the Hoffer QST, Holladay 2, Holladay 2-NLR, Kane, T2, and VRF formulas in patients with HM. Our findings may provide information on the choice, optimization, improvement, and development of IOL power formulas with high predictive accuracy for patients with HM.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169–78. https://doi.org/10.1016/j.ophtha.2017.08.027.
Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane JX. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK National Health Service. J Cataract Refract Surg. 2020;46(1):2–7. https://doi.org/10.1016/j.jcrs.2019.08.014.
Takahashi H, Tanaka N, Shinohara K, et al. Importance of paravascular vitreal adhesions for development of myopic macular retinoschisis detected by ultra-widefield OCT. Ophthalmology. 2021;128(2):256–65. https://doi.org/10.1016/j.ophtha.2020.06.063.
Takahashi H, Tanaka N, Shinohara K, et al. Ultra-widefield optical coherence tomographic imaging of posterior vitreous in eyes with high myopia. Am J Ophthalmol. 2019;206:102–12. https://doi.org/10.1016/j.ajo.2019.03.011.
Koch DD, Hill W, Abulafia A, Wang L. Pursuing perfection in intraocular lens calculations: I. Logical approach for classifying IOL calculation formulas. J Cataract Refract Surg. 2017;43(6):717–8. https://doi.org/10.1016/j.jcrs.2017.06.006.
Chong EW, Mehta JS. High myopia and cataract surgery. Curr Opin Ophthalmol. 2016;27(1):45–50. https://doi.org/10.1097/icu.0000000000000217.
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238(9):765–73. https://doi.org/10.1007/s004170000188.
Zhu X, Li D, Du Y, He W, Lu Y. DNA hypermethylation-mediated downregulation of antioxidant genes contributes to the early onset of cataracts in highly myopic eyes. Redox Biol. 2018;19:179–89. https://doi.org/10.1016/j.redox.2018.08.012.
Bor Z, Osvay K, Rácz B, Szabó G. Group refractive index measurement by Michelson interferometer. Optics Commun. 1990;78(2):109–12. https://doi.org/10.1016/0030-4018(90)90104-2.
De Bernardo M, Cione F, Capasso L, Coppola A, Rosa N. A formula to improve the reliability of optical axial length measurement in IOL power calculation. Sci Rep. 2022;12(1):18845. https://doi.org/10.1038/s41598-022-23665-0.
Lin L, Xu M, Mo E, et al. Accuracy of newer generation IOL power calculation formulas in eyes with high axial myopia. J Refract Surg. 2021;37(11):754–8. https://doi.org/10.3928/1081597x-20210712-08.
Bernardes J, Raimundo M, Lobo C, Murta JN. A comparison of intraocular lens power calculation formulas in high myopia. J Refract Surg. 2021;37(3):207–11. https://doi.org/10.3928/1081597x-20201123-01.
Chu YC, Huang TL, Chang PY, et al. Predictability of 6 intraocular lens power calculation formulas in people with very high myopia. Front Med (Lausanne). 2022;9:762761. https://doi.org/10.3389/fmed.2022.762761.
Zhou D, Sun Z, Deng G. Accuracy of the refractive prediction determined by intraocular lens power calculation formulas in high myopia. Indian J Ophthalmol. 2019;67(4):484–9. https://doi.org/10.4103/ijo.IJO_937_18.
Wan KH, Lam TCH, Yu MCY, Chan TCY. Accuracy and precision of intraocular lens calculations using the new Hill-RBF version 2.0 in eyes with high axial myopia. Am J Ophthalmol. 2019;205:66–73. https://doi.org/10.1016/j.ajo.2019.04.019.
Tan Q, Lin D, Wang L, et al. Comparison of IOL power calculation formulas for a trifocal iol in eyes with high myopia. J Refract Surg. 2021;37(8):538–44. https://doi.org/10.3928/1081597x-20210506-01.
Fuest M, Plange N, Kuerten D, et al. Intraocular lens power calculation for plus and minus lenses in high myopia using partial coherence interferometry. Int Ophthalmol. 2021;41(5):1585–92. https://doi.org/10.1007/s10792-020-01684-y.
Shammas HJ, Taroni L, Pellegrini M, Shammas MC, Jivrajka RV. Accuracy of newer intraocular lens power formulas in short and long eyes using sum-of-segments biometry. J Cataract Refract Surg. 2022. https://doi.org/10.1097/j.jcrs.0000000000000958.
Savini G, Taroni L, Hoffer KJ. Recent developments in intraocular lens power calculation methods-update 2020. Ann Transl Med. 2020;8(22):1553. https://doi.org/10.21037/atm-20-2290.
Jeong J, Song H, Lee JK, Chuck RS, Kwon JW. The effect of ocular biometric factors on the accuracy of various IOL power calculation formulas. BMC Ophthalmol. 2017;17(1):62. https://doi.org/10.1186/s12886-017-0454-y.
Yoon CH, Seol BR, Choi HJ. Effect of pterygium on corneal astigmatism, irregularity and higher-order aberrations: a comparative study with normal fellow eyes. Sci Rep. 2023;13(1):7328. https://doi.org/10.1038/s41598-023-34466-4.
Cione F, De Bernardo M, Gioia M, et al. A no-history multi-formula approach to improve the IOL power calculation after laser refractive surgery: preliminary results. J Clin Med. 2023. https://doi.org/10.3390/jcm12082890.
Zhang D, Yang M, Liu Z, Cai H, Chen X, Zhang C. The effect of implantable collamer Lens V4c on ocular biometric measurements and intraocular lens power calculation based on Pentacam-AXL and IOLMaster 500. BMC Ophthalmol. 2022;22(1):421. https://doi.org/10.1186/s12886-022-02644-z.
Zhang J, Han X, Chen X, et al. Choice of intraocular lens calculation formula for cataract patients with prior pars plana vitrectomy. J Cataract Refract Surg. 2023;49(9):956–63. https://doi.org/10.1097/j.jcrs.0000000000001253.
Frings A, Dulz S, Skevas C, et al. Postoperative refractive error after phacovitrectomy for epiretinal membrane with and without macular oedema. Graefes Arch Clin Exp Ophthalmol. 2015;253(7):1097–104. https://doi.org/10.1007/s00417-015-2948-8.
Savini G, Hoffer KJ, Balducci N, Barboni P, Schiano-Lomoriello D. Comparison of formula accuracy for intraocular lens power calculation based on measurements by a swept-source optical coherence tomography optical biometer. J Cataract Refract Surg. 2020;46(1):27–33. https://doi.org/10.1016/j.jcrs.2019.08.044.
Voytsekhivskyy OV. Development and clinical accuracy of a new intraocular lens power formula (VRF) compared to other formulas. Am J Ophthalmol. 2018;185:56–67. https://doi.org/10.1016/j.ajo.2017.10.020.
Savini G, Hoffer KJ, Barboni P. Influence of corneal asphericity on the refractive outcome of intraocular lens implantation in cataract surgery. J Cataract Refract Surg. 2015;41(4):785–9. https://doi.org/10.1016/j.jcrs.2014.07.035.
Hoffer KJ, Savini G. Update on intraocular lens power calculation study protocols: the better way to design and report clinical trials. Ophthalmology. 2021;128(11):e115–20. https://doi.org/10.1016/j.ophtha.2020.07.005.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. https://doi.org/10.3758/bf03193146.
Miao A, Tang Y, Zhu X, Qian D, Zheng T, Lu Y. Associations between anterior segment biometry and high axial myopia in 3438 cataractous eyes in the Chinese population. BMC Ophthalmol. 2022;22(1):71. https://doi.org/10.1186/s12886-022-02300-6.
Wei L, He W, Meng J, Qian D, Lu Y, Zhu X. Evaluation of the white-to-white distance in 39,986 Chinese cataractous eyes. Invest Ophthalmol Vis Sci. 2021;62(1):7. https://doi.org/10.1167/iovs.62.1.7.
Bikbov MM, Kazakbaeva GM, Gilmanshin TR, et al. Axial length and its associations in a Russian population: the Ural Eye and Medical Study. PLoS ONE. 2019;14(2):e0211186. https://doi.org/10.1371/journal.pone.0211186.
Jin G, Liu Z, Wang L, Zhu Y, Luo L, Liu Y. Corneal biometric features and their association with axial length in high myopia. Am J Ophthalmol. 2022;238:45–51. https://doi.org/10.1016/j.ajo.2021.11.031.
Liu X, Ye H, Zhang Q, et al. Association between myopia, biometry and occludable angle: the Jiangning Eye Study. PLoS ONE. 2016;11(10):e0165281. https://doi.org/10.1371/journal.pone.0165281.
Meng J, Wei L, He W, Qi J, Lu Y, Zhu X. Lens thickness and associated ocular biometric factors among cataract patients in Shanghai. Eye Vis (Lond). 2021;8(1):22. https://doi.org/10.1186/s40662-021-00245-3.
Satou T, Shimizu K, Tsunehiro S, et al. Relationship between crystalline lens thickness and shape and the identification of anterior ocular segment parameters for predicting the intraocular lens position after cataract surgery. Biomed Res Int. 2019;2019:3458548. https://doi.org/10.1155/2019/3458548.
Scholz K, Messner A, Eppig T, Bruenner H, Langenbucher A. Topography-based assessment of anterior corneal curvature and asphericity as a function of age, sex, and refractive status. J Cataract Refract Surg. 2009;35(6):1046–54. https://doi.org/10.1016/j.jcrs.2009.01.019.
Hashemi H, Nabovati P, Aghamirsalim M, Mahboubipour H, Yekta A, Khabazkhoob M. Corneal asphericity and related factors in the geriatric population: a population-based study. Ophthalmic Physiol Opt. 2021;41(4):691–701. https://doi.org/10.1111/opo.12831.
Zhang Z, Wang J, Niu W, et al. Corneal asphericity and its related factors in 1052 Chinese subjects. Optom Vis Sci. 2011;88(10):1232–9. https://doi.org/10.1097/OPX.0b013e31822717ca.
Satou T, Shimizu K, Tsunehiro S, et al. Development of a new intraocular lens power calculation method based on lens position estimated with optical coherence tomography. Sci Rep. 2020;10(1):6501. https://doi.org/10.1038/s41598-020-63546-y.
Acknowledgements
We thank Dr. David L. Cooke (Great Lakes Eye Care, St. Joseph, Michigan, USA), Dr. Jack X. Kane (Royal Victorian Eye and Ear Hospital, Melbourne, Australia), and Dr. Tun Kuan Yeo (National Healthcare Group Eye Institute, Tan Tock Seng Hospital, Singapore, Singapore), who independently analyzed our data and provided optimized lens constants.
Funding
National Natural Science Foundation of China (81970780, 81670835, and 82101103); Scientific Research Projects of the Shanghai Science and Technology Commission (19ZR1408600 and 22ZR1410400); and Shanghai Shenkang Hospital Development Center/Joint Research Project of New Frontier Technology in Municipal Hospitals (SHDC12020111). Dr. Tianyu Zheng is funding the journal’s Rapid Service fee.
Author information
Authors and Affiliations
Contributions
Ao Miao, Yi Lu and Tianyu Zheng designed the study. Ao Miao, Peimin Lin, Shaolong Ren, Jie Xu, Fan Yang, and Dongjin Qian performed the study. Ao Miao and Peimin Lin managed the data. Ao Miao, Peimin Lin, Shaolong Ren, Fan Yang, and Dongjin Qian analyzed and interpreted the data. Ao Miao and Peimin Lin wrote the initial draft. Ao Miao and Tianyu Zheng revised the manuscript. Yi Lu and Tianyu Zheng supervised the study. All authors provided a final review and approved the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of Interest
Ao Miao, Peimin Lin, Shaolong Ren, Jie Xu, Fan Yang, Dongjin Qian, Yi Lu, and Tianyu Zheng declare that they have no competing interests.
Ethical Approval
This study was approved by the Medical Ethics Committee of the Eye & ENT Hospital (Fudan University), Shanghai, China. Informed consent to participate in the study was obtained from all patients included in the study. The research protocols adhered to the tenets of the Declaration of Helsinki and its subsequent revisions.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Miao, A., Lin, P., Ren, S. et al. Influence of Ocular Biometry Parameters on the Predictive Accuracy of IOL Power Formulas in Patients with High Myopia. Ophthalmol Ther 13, 435–448 (2024). https://doi.org/10.1007/s40123-023-00856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00856-0