Abstract
Glaucoma remains a leading cause of blindness globally. Minimally invasive treatment techniques are rapidly expanding the availability of therapeutic options for glaucoma. These include devices aimed at enhancing outflow through the subconjunctival space, Schlemm’s canal, and suprachoroidal space, sustained-release drug delivery devices, and extraocular devices aiming to reduce glaucomatous progression through other novel means. In this review, we provide an overview of several novel devices either newly available or in development for the medical and surgical management of glaucoma. Further studies are required to determine the long-term efficacy of these devices and how they will integrate into the current landscape of glaucoma management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
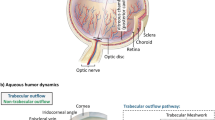
The management of glaucoma involves medical and surgical approaches aimed at lowering intraocular pressure, achieved by either increasing aqueous outflow through trabecular and/or uveoscleral pathways or decreasing aqueous production. |
Minimally invasive treatment techniques are rapidly broadening the therapeutic options available for glaucoma, which include devices aimed at enhancing outflow through the subconjunctival space, Schlemm’s canal, and suprachoroidal space, sustained-release drug delivery devices, and extraocular devices aimed at reducing glaucoma progression through other novel means. |
This review provides an overview of devices either newly available or in development for the medical and surgical treatment of glaucoma. |
Additional studies are required to determine the long-term efficacy of these devices and their role in glaucoma management. |
Introduction
Glaucoma is a leading cause of irreversible blindness globally [1]. The goal of treatment is the reduction of intraocular pressure (IOP) by either increasing aqueous egress through trabecular and/or uveoscleral outflow or by reducing aqueous production. Medical management is often limited by poor adherence, polypharmacy, as well as local and systemic side effects. Extended-release drug delivery devices could contribute to overcoming some of these limitations. Laser trabeculoplasty may be used as first-line therapy or for those in whom conservative medical treatments are inadequate but is limited by efficacy and repeatability. For patients who fail laser and medical treatment, surgical interventions are often required. In addition to trabeculectomy and glaucoma drainage devices, recent advancements in minimally invasive glaucoma surgical (MIGS) devices have expanded therapeutic options available to patients and providers. This review aims to provide an overview of novel devices in development for the medical and surgical management of glaucoma. The devices included in this review satisfy one or more of the following criteria: (1) they are described in peer-reviewed publication(s); (2) they are currently in human clinical trials either abroad or in the US; (3) they were highlighted at the 2022 American Academy of Ophthalmology symposium titled “New Devices in Glaucoma.” This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Enhancing Aqueous Outflow Through the Subconjunctival Space
In general, MIGS devices and procedures are designed for the treatment of mild to moderate open-angle glaucoma and do not typically offer IOP-lowering effects on par with traditional filtration devices and procedures. However, trabeculectomy and traditional large-lumen tube shunts require more extensive tissue manipulation and carry a higher risk of vision-threatening complications. The resulting filtering bleb can also cause issues with tear distribution, dysethesias, diplopia, ptosis, and other cosmetic concerns. Ultimately, drainage blebs can fail over time in large partbecause of the formation of a fibrotic capsule limiting aqueous outflow and absorption. Revisions including bleb needling can only offer limited success after bleb failure. Several novel bleb-forming subconjunctival drainage devices aim to improve long-term efficacy by making improvements upon the design and materials used in this space. The Xen-45 gel stent (Abbvie/Allergan, Irvine, CA, USA) remains the only device approved in the US that utilizes this approach.
The XEN gel stent is a 6-mm flexible tube with a 45-µm inner lumen diameter made of porcine collagen-derived gelatin cross-linked with glutaraldehyde, designed for ab interno implantation to create a permanent shunt between the anterior chamber and the subconjunctival space. It received Food and Drug Administration (FDA) approval in 2016. A meta-analysis showed that it can reduce IOP by 35% to a final mean value of 15 mmHg [2]. In a recent randomized contolled trial of 158 eyes, the gel stent was statisically noninferior to trabeculectomy in achieving ≥ 20% IOP reduction from baseline without medication increase [3]. However, its utility may be limited by poor long-term efficacy compared to tube shunts, often requiring revisions such as needling [4]. A promising modified ab externo approach demonstrates comparable safety and efficacy profiles, which may require less postoperative needling compared to ab interno implantation [2, 5, 6]. Recently, a larger lumen XEN-63 device has been commercialized outside the US, with preliminary findings showing improved efficacy compared to XEN-45 [7].
The PreserFlo Microshunt is an 8.5-mm tube with a 70-μm inner lumen diameter composed of the synthetic polymer poly(styrene-block-isobutylene-styrene) and designed to be implanted ab externo forming a bleb under the conjunctiva and Tenon’s capsule. A meta-analysis of over a thousand PreserFlo microshunt devices showed an average IOP reduction from 22 mmHg preoperatively to 11 mmHg postoperatively after 3 years as well as reductions in ocular hypotensive medication use [8]. In a 2-year prospective randomized clinical trial, PreserFlo was inferior to trabeculectomy at reducing IOP but had reduced risk for hypotony [9]. This device received the CE Mark in 2012 but has not received FDA approval [10].
VisiPlate (Avisi Technologies, Philadelphia, PA, USA)
VisiPlate is a flexible, ultrathin device that consists of a 400-nm aluminum oxide plate coated with a 2-µm-thick layer of parylene-C, designed to create a more physiologic bleb (Fig. 1). The structure of the device consists of a series of hexagons with intervening channels designed to provide slow, controlled outflow to create a diffuse low-lying bleb, which both prevents hypotony and results in greater comfort for the patient compared to an elevated bleb. Limited animal studies were conducted to demonstrate the preliminary safety and efficacy of the device [11]. First in human studies are currently underway.
Visiplate aqueous shunt [73]. A Image of the device (balanced on the finger tip) demonstrating its profile. B The material consists of microchannels intended to create multiple outflow pathways while maintaining resistance to prevent hypotony. C Serial optical coherence tomography images of an implant in a human eye. The device remained in position through month 3, extending from the anterior chamber to the subconjunctival space with a shallow bleb overlying the plate
GORE Glaucoma Drainage Implant (GORE GDI, W.L. Gore & Associates, Newark, DE, USA)
The GORE GDI is a 100-μm-thick device consisting of a bilayered pocket of expanded polytetrafluoroethylene (ePTFE) material connected to a silicone tube that is inserted into the anterior chamber [12] (Fig. 2). ePTFE is a patented GORE material that has been used in other biomedical devices including vascular grafts [13] and hernia membranes [14]. It is designed to encourage physiologic tissue integration following implantation, resulting in a thinner, less dense fibrotic capsule than those formed over current glaucoma drainage implants, potentially allowing for improved aqueous permeability and sustained IOP-lowering. Preclinical work in rabbit models has shown a thinner and more permeable capsule associated with the GORE GDI compared to that associated with identically sized traditional silicone drainage implants in the same model system [15]. The study was not powered to detect a difference in IOP lowering between the GORE GDI and silicone controls. Human clinical trials are underway with clinical data expected in 2023 [16].
GORE glaucoma drainage implant (GDI) concept [12]. A Current design of the GORE GDI, with a tube connected to an inflatable reservoir composed of proprietary bilayered GORE ePTFE. B Electron microscopy of a sheet of the bilayered ePTFE. The external facing surface is designed to facilitate tissue ingrowth and is backed by an aqueous permeable cell exclusion layer. C Two sheets of the bilayered material are joined and sealed to form a pocket. Aqueous is shunted into the reservoir via the tube and then percolates through the GORE material into the surrounding tissues
Minimally Invasive Micro Sclerostomy (MIMS, Sanoculis, Kiryat Ono, Isreal)
MIMS is an ab interno stent-less procedure that uses an automated Micro-Trephine to remove a 90-μm diameter cylinder of scleral tissue and creates a drainage channel from the anterior chamber to the subconjunctival space (Fig. 3). It was granted CE marking in 2017. A study from India showed a 47.5% IOP reduction from baseline at the primary endpoint of 24 weeks in a cohort who underwent either MIMS alone or MIMS-phacoemulsification [17]. Iris clogging requiring laser or trabeculectomy was the most common complication in the study, seen in 16% of subjects and thought to be due to hypotony from excessive filtration through the channel. According to the company, a more recent European study demonstrated 38% IOP reduction from baseline without major complications in 93 out of 120 patients who completed one year of follow up. A US clinical trial has not provided published results at the time of this publication [18]. Sanoculis has revealed plans for US commercial launch in 2025 [19].
Minimally invasive micro-sclerostomy (MIMS) procedure [74, 75]. A Mitomycin C and viscoelastic are delivered to the subconjunctival space to the area where aqueous will be shunted via the sclerotomy. B A side port is created, and the the anterior chamber is filled with viscoelastic. C The MIMS surgical device is inserted ab interno and advanced above the iris plane towards the angle. D, E The MIMS footpedal is used to create the drainage channel, and a cylinder of scleral tissue is removed, F allowing for flow from the aqueous chamber to the subconjunctival space
Enhancing Aqueous Outflow Through Schlemm’s Canal
Currently, improved drainage following trabecular meshwork (TM) bypass is achieved with stent placement, surgical excision/incision of the TM, or via dilation of Schlemm’s canal to improve the communication between the canal and the collector channels.
Commercially available TM bypass stents include the iStent Inject and iStent Infinite (Glaukos, Aliso Viejo, CA, USA), with two (Inject) or three (Infinite) circular microstents in the TM [20], and the Hydrus (Alcon, Fort Worth, TX, USA), a stent composed of nickel titanium (nitinol) [21]. Cutting devices used for TM excision include Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA, USA) [22] and Trabectome (NeoMedix Corp., San Juan Capistrano, CA, USA) [23]. Gonioscopy-assisted transluminal trabeculostomy (GATT) is a technique in which the TM is circumferentially opened with either an illuminated microcatheter or a suture [24]. Ab interno canaloplasty (ABiC) uses either the iTrack microcatheter (Ellex, Adelaide, Australia) or VISCO360 (Sight Sciences, Menlo Park, CA, USA) to viscodilate Schlemm’s canal [25], whereas the OMNI surgical system (Sight Sciences, Menlo Park, CA, USA) viscodilates Schlemm’s canal and collector channels with the option of subsequent goniotomy [26]. Overall, the effectiveness of some of these devices, in particular ones in which no TM tissue is removed, may be limited by wound healing and/or scarring over time as follow-up data beyond 96 months are not yet available [27,28,29].
Excimer Laser Trabeculostomy (ELT, Elios Vision Inc., Los Angeles, CA, USA)
Developed by Berlin et al. [30], ELT is the first laser-based MIGS procedure. The device utilizes 308-nm xenon chloride excimer laser transmitted through a fiber optic probe to ablate portions of the TM and create macrochannels to Schlemm’s canal via an intracameral approach (Fig. 4). In contrast to selective laser trabeculoplasty, which used a 532-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser to deliver thermal radiation to the TM, ELT uses a “cold” laser that theoretically minimizes thermal damage. Decreased scar formation and inflammatory response might be expected given the non-thermal approach and the lack of foreign devices implanted, although this remains to be seen. A rabbit study showed an absence of fibroblast migration up to 5 weeks after ELT, which would theoretically limit postoperative scarring [31]. Existing studies demonstrated an IOP-lowering between 20 and 40% from baseline without prior medication washout and a relatively low number of complications, resulting in fewer glaucoma medications [32]. The procedure can be performed alone or in conjunction with phacoemulsification [32] and has been approved for use in the European Union and Switzerland since 1998. ELT is currently in a US clinical trial with an estimated study completion time of 2024 [33].
Excimer laser trabeculostomy [73]. A A probe applies laser energy directly to the trabecular meshwork using a foot pedal system. B Blood and microbubbles are often seen post-intervention
Enhancing Aqueous Outflow Through the Suprachoroidal Space
Accessing the suprachoroidal space by safely generating a cyclodialysis cleft to increase uveoscleral outflow has long been an area of interest within glaucoma surgery. The large surface area and negative pressure gradient of the suprachoroidal space provide a good driving force for aqueous drainage, while cyclodialysis clefts are well known to have significant IOP-lowering effects clinically [34]. Several first-generation devices have validated this approach but demonstrated side effects that ultimately limited their clinical applications.
The Cypass micro-stent (ALCON, Fribourg, Switzerland) was the first ab interno suprachoroidal drainage device to receive FDA approval in 2017. The COMPASS trial for the micro-stent showed promising sustained IOP-lowering effects at 2 years [35], but the device, due to its rigidity and positioning relative to the cornea, led to long-term corneal endothelial cell loss. In the 5-year follow-up analysis, subjects who underwent device implantation plus phacoemulsification demonstrated a 20.4% mean decrease in corneal endothelial cell density compared to 10.1% decrease in subjects who underwent phacoemulsification alone [36]. Cypass has since been voluntarily withdrawn from the market by the manufacturer.
The Gold Micro Shunt Plus (SOLX Ltd., Waltham, MA, USA) was an implant made of 24-carat gold with microchannels which ultimately failed to demonstrate efficacy in IOP reduction because of significant fibrosis around the device and its micropores [37].
The iSTENT Supra (GLAUKOS, San Clemente, CA, USA) is a 4-mm-long tube composed of polyethersulfone and titanium [38]. In a 2018 study by Myers et al., the iSTENT Supra demonstrated IOP control comparable to two iStent trabecular micro-bypass stents and postoperative prostaglandin following trabeculectomy at 4 years [39]. This device received the European Union CE Mark in 2010, and a randomized clinical trial in the US was completed in 2020 with no published results at this time [40].
MINIject (ISTAR Medical, Wavre, Belgium)
The MINIject is a 5-mm-long uveoscleral device consisting of proprietary Star silicone material designed to be microporous and flexible for ab interno implantation [41] (Fig. 5). Two-year outcomes in a study of 25 patients showed a 40% IOP reduction from baseline with all patients achieving at least 20% IOP reduction [42]. In this study, the device was shown to be well tolerated, with a 5% decrease in mean central endothelial cell density. Three prospective, single-arm trials totaling 66 patients showed consistent positive results at 2 years [43]. The device has been commercially available in Europe since November 2021, and the Star-V US trial is currently enrolling subjects with an estimated primary completion date of 2025 [44].
iSTAR MINIject [73]. A The device is 5 mm × 1 mm and made of proprietary silicone material. B Final placement of device in the suprachoroidal space (red arrow). Proper placement is achieved when the green ring is at the level of scleral spur
Suprachoroidal Bio-tissue Device (IANTREK, White Plains, NY, USA)
This biostent is a permeable scleral allograft of homologous acellular matrix designed to minimize the negative effects of synthetic foreign material in the eye [45] (Fig. 6). Preliminary 12-month results of ten subjects showed a statically significant 40% reduction of IOP from baseline. The device demonstrated an 11% endothelial cell loss at 12 months after combined phaco-biostenting surgery. Manufacturers are currently planning a larger study involving multiple sites globally to further assess the safety and efficacy of the device [45].
Iantrek biostent [73]. A The implant is inserted into a cyclodialysis cannula for ab interno delivery. B Once deployed, the biostent reinforces an iatrogenic cyclodialysis cleft, allowing for drainage around and through the porous device into the suprachoroidal space
Sustained-Release Drug Delivery Devices
To mitigate issues of non-adherence and ocular surface side effects, a variety of sustained-release drug delivery systems are being considered as alternatives to topical delivery of IOP-lowering medications requiring daily dosing. At the time of this publication, Durysta (Allergen, Dublin, Ireland), a bimatoprost intraocular implant, remains the only US FDA-approved sustained release therapy for primary open angle glaucoma. Compared to topical timolol, the bimatoprost intraocular implant was found to meet pre-defined criteria for non-inferiority after 12 weeks [46, 47]. Durysta was also found to be comparable to timolol with respect to IOP lowering in the 12 weeks after a second and a third injection. A Kaplan-Meier survival analysis estimated a probability of not requiring additional treatment for 1 year after the last implant injection to be in the range of 70–75%. Based on trial results, the bimatoprost implant 10 μg was approved for a single intracameral administration for IOP control in patients with open-angle glaucoma and ocular hypertension.
The bimatoprost ocular ring (Allergan, Dublin, Ireland) is placed in the upper and lower fornices and consists of an inner polypropylene support ring with an outer silicone matrix containing bimatoprost. A phase II trial did not meet pre-defined noninferiority criteria compared to topical timolol at seven of nine time points over the course of 6 months [48]. The study was also underpowered for the observed treatment effect.
ENV515 (Envisia Therapeutics, Research Triangle Park, NC, USA) is an implant placed in the iridocorneal angle designed to release travoprost over the course of 6–12 months. Data from a phase II clinical trial reported a mean IOP decrease of ~ 25% over 11 months [49, 50].
OTX-TP (Ocular Therapeutix, Bedford, MA, USA) (NCT02914509) is a resorbable hydrogel-based punctal plug designed to deliver travoprost to the ocular surface over a 3-month period. A prospective, phase III trial showed statistically significant IOP reduction compared to placebo at eight out of nine time points ranging from 3 to 6 mmHg [51].
The OTX-TIC is another travoprost bioresorbable implant from Ocular Therapeutix that is designed for intracameral insertion. Phase I results showed an average IOP reduction of 7–10 mmHg from baseline [52]. Implants were biodegraded within 3–7 months depending on the composition of the implant. Subjects are currently being recruited for a phase II clinical trial [53].
PA5108 and PA5346 (PolyActiva, Parkville, Australia) are two biodegradable devices designed to release latanoprost free acid [54]. At the Glaucoma 360 New Horizons Forum, PolyActiva reported meeting primary and secondary efficacy endpoints of > 20% IOP reduction in a low dose cohort at 12 and 26 weeks for a phase IIa clinical trial of PA5108 [54, 55]. A phase I study of the second generation PA5346 implant is ongoing [56].
iDose TR (Glaukos, Aliso Viejo, CA, USA)
The iDose TR is a 1.8 × 0.55-mm biocompatible titanium implant. The device is comprised of a scleral anchor which affixes it in the anterior chamber, a drug reservoir which contains 75 μg of a proprietary formulation of travoprost, and a retaining cap with an elution membrane that provides sustained travoprost release (Fig. 7). In a Phase IIb trial comprised of 154 patients, a single implantation of either a fast- or slow-eluting implant was compared to twice-daily topical timolol (0.5%). After 36 months, patients who received the fast- and slow-eluting implants exhibited an average of 8.3 and 8.5 mmHg reduction in IOP, respectively. Seventy and 68% of patients in the fast- and slow-eluting arms were found to be controlled on the same or fewer IOP-lowering medications. By comparison, patients in the twice-daily timolol arm demonstrated an average IOP reduction of 8.2 mmHg, with 46% of patients found to be controlled on the same or fewer medications. Based on these results, the slow-eluting implant was selected to become iDose TR [57, 58].
iDose implant [73]. A The device is anchored into the scleral tissue behind the trabecular meshwork. B Image of the implant in the anterior chamber angle nasally in a human eye.
Thirty-two subjects from the phase IIb trial were included in a separate trial to evaluate the safety and tolerability of an iDose TR exchange. Subjects were observed for corneal endothelial cell counts at baseline, after 3 years, and at 4.2 years when the device was exchanged. A final endothelial cell count was performed 1 year after the exchange. Per the company, the procedure was well tolerated with favorable safety profiles at 12 months following the second implant, and no subject exhibited > 30% endothelial cell loss over the extended evaluation period of 5.2 years [59, 60].
Most recently, two phase III trials (GC-010 and GC-012) consisting of 590 and 560 subjects achieved a pre-specified primary efficacy endpoint of noninferiority to twice-daily topical 0.5% timolol after 3 months [61,62,63]. In the GC-010 trial, IOP reductions from baseline ranged from 6.6 to 8.5 mmHg in the iDose TR arm compared to 6.6–7.7 mmHg in the timolol arm. By comparison, IOP reduction from baseline in the GC-012 arm ranged from 6.7 to 8.4 mmHg in the iDose TR arm compared to 6.8–7.2 mmHg in the timolol arm. Twelve months following treatment, approximately 93% of subjects in the iDose TR arm were assessed to be controlled with the same or fewer number of IOP-lowering topical medications compared to 67% of subjects in the timolol arm. At 12 months, 81% of iDose TR subjects were no longer taking IOP-lowering topical medications. Tolerability and safety were assessed to be good after 12 months of follow-up. The most common adverse event in the GC-010 and GC-012 trials was transient iritis in 5.5% and 6.2% of patients. Conjunctival hyperemia was reported in 2.6% of iDose TR patients compared to 0.5% in timolol treated patients. There were no reports of corneal endothelial cell loss, serious corneal adverse events, or periorbital fat atrophy. Glaukos submitted its new drug application in February 2023 for FDA approval [64].
Reducing glaucomatous Progression Through Other Means (External Devices)
Currently, there are no treatment options for glaucoma that are both non-pharmacologic and do not involve either an intra- or peri-ocular procedure or surgery. Several devices are currently in development.
Multi-pressure Dial (MPD, Equinox Ophthalmic, Inc., Newport Beach, CA, USA)
Peak IOP is thought to occur at night because of head and body positioning [65, 66]. In addition, current ocular hypotensive medications may not adequately control nocturnal IOP [67]. The MPD is a non-invasive and non-pharmaceutical approach to lowering IOP, intended for nightime use and in combination with existing IOP-lowering therapies. The device consists of a pair of removable goggles connected to a titratable pressure-modulating pump that creates a focal negative pressure environment over the orbits to reduce IOP (Fig. 8). The device would ideally titrate the level of IOP reduction by modulating the amount of negative pressure within the goggles. In a study of healthy eyes, from a baseline IOP of 16 mmHg without negative pressure, the mean IOP was 14, 12, and 10 mmHg with negative pressure settings of 25, 50, and 75%, respectively [68]. Results of a clinical trial involving 64 patients with glaucoma who remained on their IOP-lowering medications were reported at the 2022 American Academy of Ophthalmology meeting [69]. While wearing the device, 90% of study eyes met the primary endpoint of > 20% IOP reduction from baseline compared to 3% of contralateral control eyes. No serious adverse events were reported.
Multi-pressure dial (MPD) [73]. The device consists of airtight goggles attached to a titratable negative pressure pump
Eyetronic Device (Neuromodtronic GmbH, Potsdam, Germany)
Preclinical data suggests electrical optic nerve stimulation may have neurorestorative and neuroprotective effects [70]. The Eyetronic device emits electrical pulses to simulate the optic nerve using goggles with embedded supraorbital and infraorbital electrodes. After receiving the CE mark in 2016, 12-month post-market data of 70 glaucoma patients showed a halt in disease progression in 63% of eyes after 10 daily sessions of optic nerve stimulation within a 2-week period [71]. This treatment is currently available in Germany, Italy, and Switerland [72].
Conclusion
The field of minimally invasive glaucoma treatment techniques has been rapidly expanding, providing a range of options for patients and providers. These approaches encompass devices that enhance aqueous outflow through the subconjunctival space, Schlemm’s canal, and suprachoroidal space, sustained drug delivery devices, and external devices that aim to reduce glaucomatous progression through novel mechanisms. The long-term efficacy of these interventions remains to be seen, and their ultimate ability to integrate into existing glaucoma management landscape will rely on results of further investigation.
References
Tham YC, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90.
Chen XZ, et al. The outcomes of XEN gel stent implantation: a systematic review and meta-analysis. Front Med (Lausanne). 2022;9: 804847.
Sheybani A, et al. Gel Stent vs Trabeculectomy: The Randomized, Multicenter, Gold Standard Pathway Study (GPS) of effectiveness and safety at 12 months: gel stent vs trabeculectomy: a prospective randomized study. Am J Ophthalmol. 2023;252:306–25.
Midha N, et al. Efficacy of needling revision after XEN gel stent implantation: a prospective study. J Glaucoma. 2020;29(1):11–4.
Do A, et al. Comparison of clinical outcomes with open versus closed conjunctiva implantation of the XEN45 gel stent. Ophthalmol Glaucoma. 2021;4(4):343–9.
Tan NE, et al. Comparison of safety and efficacy between Ab interno and Ab externo approaches to XEN gel stent placement. Clin Ophthalmol. 2021;15:299–305.
Hussien IM, De Francesco T, Ahmed IIK. Intermediate outcomes of the novel 63 μm gelatin microstent versus the conventional 45 μm gelatin microstent. Ophthalmol Glaucoma. 2023. https://doi.org/10.1016/j.ogla.2023.05.001.
Pawiroredjo SSM, et al. Efficacy of the PRESERFLO microshunt and a meta-analysis of the literature. J Clin Med. 2022;11(23):7149.
Baker ND, et al. Ab-externo microshunt versus trabeculectomy in primary open-angle glaucoma: one-year results from a 2-year randomized, multicenter study. Ophthalmology. 2021;128(12):1710–21.
Saeed E, et al. The PreserFlo MicroShunt in the context of minimally invasive glaucoma surgery: a narrative review. Int J Environ Res Public Health. 2023;20(4):2904.
Kao BW, et al. Biocompatibility and feasibility of VisiPlate, a novel ultrathin, multichannel glaucoma drainage device. J Mater Sci Mater Med. 2021;32(12):141.
Preclinical Study: New gore glaucoma drainage implant 2022; Available from: https://www.aao.org/eyenet/academy-live/detail/preclinical-study-gore-glaucoma-drainage-implant
Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37(2):472–80.
Topart P, et al. Laparoscopic ventral hernia repair with the Goretex Dualmesh: long-term results and review of the literature. Hernia. 2005;9(4):348–52.
Bicket AK, et al. A novel bilayered expanded polytetrafluoroethylene glaucoma implant creates a permeable thin capsule independent of aqueous humor exposure. Bioeng Transl Med. 2021;6(1): e10179.
GORE Glaucoma Drainage Implant Clinical Study. https://ClinicalTrials.gov/show/NCT05557058.
Geffen N, et al. Minimally Invasive Micro Sclerostomy (MIMS) procedure: a novel glaucoma filtration procedure. J Glaucoma. 2022;31(3):191–200.
Minimally Invasive Micro Sclerostomy: performance and safety evaluation study. https://ClinicalTrials.gov/show/NCT04503590.
OIS Glaucoma Showcase Take-Home: glaucoma market poised for growth. 2021; Available from: https://ois.net/ois-glaucoma-showcase-take-home-glaucoma-market-poised-for-growth-disruption/.
Samuelson TW, et al. Prospective, randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–21.
Ahmed IIK, et al. Three-year findings of the HORIZON trial: a schlemm canal microstent for pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2021;128(6):857–65.
Iwasaki K, et al. Long-term outcomes of a kahook dual blade procedure combined with phacoemulsification in japanese patients with open-angle glaucoma. J Clin Med. 2022;11(5):1354.
Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205–8.
Grover DS, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–61.
Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;12:2149–55.
Ondrejka S, Korber N. 360 degrees ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–46.
Shah M. Micro-invasive glaucoma surgery—an interventional glaucoma revolution. Eye Vis (Lond). 2019;6:29.
Konopinska J, et al. Microinvasive glaucoma surgery: a review of schlemm’s canal-based procedures. Clin Ophthalmol. 2021;15:1109–18.
Salimi A, Watt H, Harasymowycz P. Long-term outcomes of two first-generation trabecular micro-bypass stents (iStent) with phacoemulsification in primary open-angle glaucoma: eight-year results. Eye Vis (Lond). 2021;8(1):43.
Berlin MS, et al. Excimer laser photoablation in glaucoma filtering surgery. Am J Ophthalmol. 1987;103(5):713–4.
Huang S, et al. Histopathological study of trabeculum after excimer laser trabeculectomy ab interno. Yan Ke Xue Bao. 2001;17(1):11–5.
Durr GM, et al. Current review of Excimer laser Trabeculostomy. Eye Vis (Lond). 2020;7:24.
Excimer laser trabeculostomy glaucoma treatment study. https://ClinicalTrials.gov/show/NCT04899063.
Gonzalez-Martin-Moro J, et al. Cyclodialysis: an update. Int Ophthalmol. 2017;37(2):441–57.
Vold S, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103–12.
Lass JH, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without CyPass micro-stent. Am J Ophthalmol. 2019;208:211–8.
Hueber A, et al. Retrospective analysis of the success and safety of Gold Micro Shunt implantation in glaucoma. BMC Ophthalmol. 2013;13:35.
Manasses DT, Au L. The new era of glaucoma micro-stent surgery. Ophthalmol Ther. 2016;5(2):135–46.
Myers JS, et al. Prospective Evaluation of Two iStent((R)) Trabecular Stents, One iStent Supra((R)) suprachoroidal stent, and postoperative prostaglandin in refractory glaucoma: 4-year outcomes. Adv Ther. 2018;35(3):395–407.
Multicenter Investigation of the Glaukos® Suprachoroidal stent model G3 in conjunction with cataract surgery. https://ClinicalTrials.gov/show/NCT01461278.
Grierson I, et al. A novel suprachoroidal microinvasive glaucoma implant: in vivo biocompatibility and biointegration. BMC Biomed Eng. 2020;2:10.
Denis P, et al. Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial. Br J Ophthalmol. 2022;106(1):65–70.
iSTAR Medical Presents Positive Consistent Results for MINIject® across three international glaucoma trials. June 8, 2022: Wavre, Belgium.
Medical, I. Evaluate the safety and effectiveness of iSTAR medical's MINIject™ implant for lowering intraocular pressure (IOP) in subjects with primary open-angle Glaucoma. 2021, https://ClinicalTrials.gov/show/NCT05024695.
Ianchulev T, et al. Biotissue stent for supraciliary outflow in open-angle glaucoma patients: surgical procedure and first clinical results of an aqueous drainage biostent. Br J Ophthalmol. 2023. https://doi.org/10.1136/bjo-2022-322536.
Medeiros FA, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–41.
Bacharach J, et al. Phase 3, randomized, 20-month study of the efficacy and safety of bimatoprost implant in patients with open-angle glaucoma and ocular hypertension (ARTEMIS 2). Drugs. 2021;81(17):2017–33.
Brandt JD, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology. 2016;123(8):1685–94.
Safety and efficacy of ENV515 Travoprost Extended Release (XR) in patients with bilateral ocular hypertension or primary open angle glaucoma. https://ClinicalTrials.gov/show/NCT02371746.
Mansberger SL, et al. Interim analysis of low dose ENV515 Travoprost XR with 11 month duration followed by dose escalation and 28 day efficacy evaluation of high dose ENV515. Invest Ophthalmol Vis Sci. 2017;58(8):2110–2110.
Vantipalli S, et al. Evaluation of the Safety and Efficacy of OTX-TP, an intracanalicular travoprost insert, for the treatment of patients with open-angle glaucoma or ocular hypertension: a phase 3 study. Invest Ophthalmol Vis Sci. 2020;61(7):3488–3488.
Goldstein MH, et al. Evaluating safety, tolerability and efficacy of an intracameral hydrogel-based travoprost implant in subjects with glaucoma—phase 1 trial. Invest Ophthalmol Vis Sci. 2020;61(7):4266–4266.
A study to evaluate the efficacy and safety of OTX-TIC (Travoprost) intracameral implant for patients with Open-angle Glaucoma (OAG) or Ocular Hypertension (OHT). https://ClinicalTrials.gov/show/NCT05335122.
Open label, sequential-dose study of PA5108 latanoprost FA SR ocular implant for mild-moderate Glaucoma. https://ClinicalTrials.gov/show/NCT04060758.
Hutton D. PolyActiva offers results of Phase IIa study of ocular implant. 2022; Available from: https://www.ophthalmologytimes.com/view/polyactiva-offers-results-of-phase-iia-study-of-ocular-implant.
Study of a latanoprost ocular implant for treatment of open angle glaucoma or ocular hypertension. https://ClinicalTrials.gov/show/NCT05333419.
Glaukos’ iDose®TR Demonstrates Sustained IOP Reduction and Favorable Safety Profile Over 36 Months in Phase 2b Study. 2022. https://investors.glaukos.com/investors/news/news-details/2022/Glaukos-iDoseTR-Demonstrates-Sustained-IOP-Reduction-and-Favorable-Safety-Profile-Over-36-Months-in-Phase-2b-Study/default.aspx.
Study comparing travoprost intraocular implants to timolol ophthalmic solution. https://ClinicalTrials.gov/show/NCT02754596.
Glaukos Announces Positive Results for iDose TR Exchange Trial, highlighting favorable safety and tolerability. 2023: https://investors.glaukos.com/investors/news/news-details/2023/Glaukos-Announces-Positive-Results-for-iDose-TR-Exchange-Trial-Highlighting-Favorable-Safety-and-Tolerability/default.aspx.
Study of exchange of travoprost intraocular implant. https://ClinicalTrials.gov/show/NCT04615403.
Randomized study comparing two models of a travoprost intraocular implant to timolol maleate ophthalmic solution, 0.5%. https://ClinicalTrials.gov/show/NCT03519386.
Clinical study comparing two models of a travoprost intraocular implant. https://ClinicalTrials.gov/show/NCT03868124.
Glaukos announces positive topline outcomes for both phase 3 pivotal trials of iDose TR, achieving primary efficacy endpoints and demonstrating favorable tolerability and safety profiles. 2022. https://investors.glaukos.com/investors/news/news-details/2022/Glaukos-Announces-Positive-Topline-Outcomes-for-Both-Phase-3-Pivotal-Trials-of-iDose-TR-Achieving-Primary-Efficacy-Endpoints-and-Demonstrating-Favorable-Tolerability-and-Safety-Profiles/default.aspx.
Glaukos submits new drug application to US FDA for iDose TR. 2023.
Malihi M, Sit AJ. Effect of head and body position on intraocular pressure. Ophthalmology. 2012;119(5):987–91.
Aref AA. What happens to glaucoma patients during sleep? Curr Opin Ophthalmol. 2013;24(2):162–6.
Gulati V, et al. Diurnal and nocturnal variations in aqueous humor dynamics of patients with ocular hypertension undergoing medical therapy. Arch Ophthalmol. 2012;130(6):677–84.
Swan RJ, et al. Evaluation of the IOP-lowering effect of a multi-pressure dial at different negative pressure settings. Transl Vis Sci Technol. 2020;9(12):19.
Negative pressure applied by the equinox MPD for severe open angle glaucoma. https://ClinicalTrials.gov/show/NCT04632329.
Rahmatnejad K, et al. Non-invasive electrical stimulation for vision restoration: dream or reality? Expert Rev Ophthalmol. 2016;11(5):325–7.
Erb C, et al. Electrical neurostimulation in glaucoma with progressive vision loss. Bioelectron Med. 2022;8(1):6.
Eyetronic restoring vision.
New Devices in Glaucoma. in American Academy of Ophthalmology. 2022. Chicago, IL.
MIMS – An innovative new Surgical procedure for Glaucoma. 2021; Available from: https://www.clinicalservicesjournal.com/story/35655/mims-an-innovative-new-surgical-procedure-for-glaucoma.
Grover DS. Minimally Invasive Micro Sclerostomy. 2022; Available from: https://glaucomatoday.com/articles/2022-sept-oct/minimally-invasive-micro-sclerostomy?c4src=home:feed.
Acknowledgements
Author Contributions
Lilian Chan: draft manuscript preparation, reviewing and editing. Marlene R Moster: manuscript review and edits, provided figure images. Amanda Bicket: manuscript review and edits, provided figure images. Arsham Sheybani: manuscript review and edits, provided figure images. Steven R Sarkisian: manuscript review and edits, provided figure images. Thomas W Samuelson: manuscript review and edits, provided figure images. Iqbal Ike K Ahmed: manuscript review and edits, provided figure images. Eydie Miller-Ellis: manuscript review and edits, provided figure images. Oluwatosin U Smith: manuscript conception and design, reviewing and editing manscript. Qi N Cui: manuscript conception and design, draft manuscript preparation, reviewing and editing.
Funding
Qi N Cui is funded by: NIH/NEI R01EY034115 (QNC), Research to Prevent Blindness. No funding or sponsorship was received for the publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Conflict of Interest
Lilian Chan and Qi N Cui have nothing to disclose. Marlene R Moster: Abbvie: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. ArcScan: Advisory Board. AeriePharmaceuticals: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. AlconLaboratories, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allergan: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Bausch + Lomb: Lecture Fees/Speakers Bureau, Grant Support. Glaukos: Grant Support. InnFocus: Grant Support. Iridex: Lecture Fees, Grant Support. MedEdicus: Lecture Fees/Speakers Bureau. NewWorldMedical, Inc.: Grant Support. Nicox: Grant Support Wills Eye. Novartis, AlconPharmaceuticals: Lecture Fees/Speakers Bureau. Regeneron: Advisor. Santen, Inc.: Consultant/Advisor, Grant Support. Thea Inc.: Grant Support, Advisor. W. L. Gore & Associates, Inc.: Advisor. Amanda Bicket: W.L. Gore & Associates: Consultant. Arsham Sheybani: Alcon, Abbvie, Nova Eye, and Glaukos: Consultant. Steven R Sarkisian: Alcon Laboratories, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allergan, Inc.: Consultant/Advisor, Lecture Fees/Speakers Bureau, Grant Support. Allysta Pharmaceuticals: Grant Support. Bausch + Lomb: Consultant/Advisor, Lecture Fees/Speakers Bureau. Beaver-Visitec International, Inc.: Consultant/Advisor. Elios: Grant Support. Glaukos Corporation: Consultant/Advisor, Grant Support. Icare USA, Inc.: Consultant/Advisor. iSTAR Medical: Grant Support. Katena Products, Inc: Consultant/Advisor. MicroSurgical Technology: Consultant/Advisor. Ocular Science: Consultant/Advisor, Grant Support, Stock Options—Public or Private Corp. Ocular Therapeutix: Grant Support. Santen, Inc.: Consultant/Advisor. Sight Sciences, Inc.: Consultant/Advisor, Grant Support, Equity/Stock Holder—Public Corp. Thomas W Samuelson: Equinox: consultant, advisory board member. Iqbal Ike K Ahmed: Aequus: Consultant/Consulting Fees. Ace Vision: Consultant/Consulting Fees. Aerie Pharmaceuticals: Consultant/Consulting Fees, Research Grant/Support. Akorn: Consultant/Consulting Fees. Alcon: Consultant/Consulting Fees,Speakers Honoraria, Research Grant/Support. Allergan: Consultant/Consulting Fees,Speakers Honoraria, Research Grant/Support. Aquea Health, Inc: Consultant/Consulting Fees. ArcScan: Consultant/Consulting Fees. Avellino Lab USA, Inc: Consultant/Consulting Fees. Avisi: Consultant/Consulting Fees. Bausch Health: Consultant/Consulting Fees. Beaver Visitec: Consultant/Consulting Fees. Belkin Vision: Consultant/Consulting Fees. Beyeonics: Consultant/Consulting Fees. Bionode: Consultant/Consulting Fees, Research Grant/Support. Carl Zeiss Meditec: Consultant/Consulting Fees, Speakers Honoraria. Centricity Vision, Inc: Consultant/Consulting Fees. CorNeat Vision: Consultant/Consulting Fees. Custom Surgical: Consultant/Consulting Fees. Elios Vision: Consultant/Consulting Fees. ElutiMed: Consultant/Consulting Fees. Equinox: Consultant/Consulting Fees. EyeFlow, Inc: Consultant/Consulting Fees. EyedMed: Consultant/Consulting Fees. EyeQ Technologies: Consultant/Consulting Fees. Exhaura Limited: Consultant/Consulting Fees. Genentech: Consultant/Consulting Fees. Glaukos: Consultant/Consulting Fees, Research Grant/Support. Gore: Consultant/Consulting Fees. Heine: Consultant/Consulting Fees, Speakers Honoraria. Heru: Consultant/Consulting Fees. Hexiris Pharma: Consultant/Consulting Fees. Iantrek: Consultant/Consulting Fees. InjectSense: Consultant/Consulting Fees. Iridex: Consultant/Consulting Fees. iCare: Research Grant/Support. iStar: Consultant/Consulting Fees. Ivantis: Consultant/Consulting Fees, Research Grant/Support. Johnson & Johnson Vision: Consultant/Consulting Fees, Speakers Honoraria, Research Grant/Support. Labtician Thea: Consultant/Consulting Fees. LayerBio: Consultant/Consulting Fees. Leica Microsystems: Consultant/Consulting Fees. Life Long Vision: Consultant/Consulting Fees. Long Bridge Medical, Inc: Consultant/Consulting Fees. MicroOptx: Consultant/Consulting Fees. MST Surgical: Consultant/Consulting Fees, Speakers Honoraria. Myra Vision/Shifamed LLC: Consultant/Consulting Fees. New World Medical: Consultant/Consulting Fees, Research Grant/Support. NovaEye: Consultant/Consulting Fees. Ocular Instruments: Consultant/Consulting Fees. Ocular Therapeutix: Consultant/Consulting Fees. Oculo: Consultant/Consulting Fees. Oculus Surgical: Consultant/Consulting Fees. Omega Ophthalmics: Consultant/Consulting Fees. PolyActiva: Consultant/Consulting Fees. PulseMedica: Consultant/Consulting Fees. Radiance Therapeutics, Inc: Consultant/Consulting Fees. Radius XR: Consultant/Consulting Fees. Rheon Medical SA: Consultant/Consulting Fees. Ripple Therapeutics: Consultant/Consulting Fees. Samsara Vision: Consultant/Consulting Fees. Sanoculis: Consultant/Consulting Fees. Santen: Consultant/Consulting Fees, Research Grant/Support. Singapore Biodesign Programme Office. Sight Sciences: Consultant/Consulting Fees. Smartlens, Inc: Consultant/Consulting Fees. Stroma: Consultant/Consulting Fees. Thea Pharma: Consultant/Consulting Fees. TFS Health Science: Consultant/Consulting Fees. ViaLase: Consultant/Consulting Fees. Visus Therapeutics: Consultant/Consulting Fees. Vizzario: Consultant/Consulting Fees. VSY Biotechnology: Consultant/Consulting Fees. Zilia, Inc: Consultant/Consulting Fees. Eydie Miller-Ellis: Avisi: scientific advisor. Aerie Pharmaceuticals, Allergan, Eyenovia, and Thea Pharama: consultant. Thea Pharma and NIH/NEI: research support. Oluwatosin U Smith: Alcon, Iridex, Santen, New World Medical, Glaukos, and Iris Vision: consultant. Aerie, Bauch and Lomb, and Allergan: speaker/consultant. Nova Eye Medical and Olleyes: stock holder. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chan, L., Moster, M.R., Bicket, A.K. et al. New Devices in Glaucoma. Ophthalmol Ther 12, 2381–2395 (2023). https://doi.org/10.1007/s40123-023-00780-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00780-3