Abstract
Introduction
To compare the visual outcomes of astigmatism correction with implantable collamer lens (ICL) surgery with low-to-moderate astigmatism through a steep-meridian corneal relaxing incision (SM–CRI) and non-steep-meridian corneal relaxing incision (NSM–CRI).
Methods
Seventy eyes of 70 patients with myopia and myopic astigmatism who underwent ICL V4c implantation were classified into two groups: SM–CRI and NSM–CRI. Refractive outcomes and vector analysis were evaluated preoperatively and 6 months postoperatively.
Results
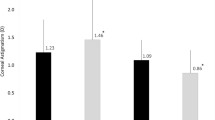
At the postoperative 6 month visit, all participants in both groups achieved an uncorrected distance visual acuity (UDVA) of 20/20 or better. The difference vector (DV) showed that the residual astigmatism in the SM–CRI group was much smaller than that in the NSM–CRI group (P = 0.021), and the correction index (CI) was 0.84 ± 0.30 and 0.67 ± 0.35 for the SM–CRI and NSM–CRI groups, respectively, with a significant statistical difference (P = 0.013). Approximately 71% of eyes in the SM–CRI group had an angle of error (AE) within ± 15°, whereas 55% of eyes in the NSM–CRI group were within that range. The absolute mean AE was 10.13 ± 14.57° in the SM–CRI group, compared with 23.88 ± 28.22° in the NSM-CRI group (P = 0.038).
Conclusion
SM–CRI can alleviate corneal astigmatism and decrease the cylindrical diopter of the ICL, thus improving postoperative visual quality compared with NSM–CRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The steep-meridian corneal relaxing incision (SM–CRI) has been widely used in cataract surgery to correct low-to-moderate corneal astigmatism. However, there is no consensus on the type of incision that should be used for implantable collamer lens (ICL) implantation to correct the myopia with low-to-moderate astigmatism. |
This study aimed to evaluate the efficacy of SM–CRI and non-steep-meridian corneal relaxing incision (NSM–CRI) in ICL surgery for low-to-moderate astigmatism correction. |
What was learned from this study? |
SM–CRI can alleviate corneal astigmatism and decrease the cylindrical diopter of the ICL, thus improving postoperative visual quality compared with NSM–CRI. |
SM–CRI might be ideal for ICL surgery for low-to-moderate astigmatism correction. |
Introduction
Currently, the toric implantable collamer lens (TICL) is widely considered a safe and effective procedure for correcting myopia and astigmatism [1,2,3,4,5]. However, its correction efficacy for low astigmatism within 1.0 D is affected by several factors. The TICL power calculation software provided by the manufacturer does not consider surgically induced astigmatism (SIA) when predicting postoperative refractive outcomes [2], and rotation can significantly reduce the correction efficacy of TICL in astigmatism [5]. The influence of these factors on astigmatism correction may reduce patient visual quality and satisfaction, particularly for low astigmatism. Therefore, it is worth exploring a simpler, safer, and more economical method to correct pre-existing low astigmatism during ICL implantation.
The steep-meridian corneal relaxing incision (SM–CRI) is made at the steep meridian of the cornea [6]. The basic principle is that the relaxing incision of the cornea flattens the steep meridian and steepens the flat meridian to reduce corneal astigmatism. This simple and economical surgical approach has been widely used in cataract surgery to correct low-to-moderate corneal astigmatism [7]. In a study by He et al. concerning the SM–CRI, they found that postoperative astigmatism showed good correction efficacy in patients with preoperative corneal astigmatism between 0.37 D and 1.0 D [8]. He et al. also found that compared with patients with non-steep-meridian corneal relaxing incision (NSM–CRI), patients with SM-CRI had significantly lower postoperative corneal astigmatism and incidence of postoperative irregular astigmatism, and better postoperative visual quality. They suggested that SM–CRI might be an ideal option for toric IOL implantation [8].
To date, there is no consensus on the type of incision that should be used for ICL implantation. Therefore, this prospective study sought to evaluate the efficacy of SM–CRI and NSM–CRI in ICL surgery for low-to-moderate astigmatism correction.
Methods
Patients
In this prospective cohort study, we recruited individuals with myopia or myopic with astigmatism who underwent ICL V4c implantation at the Eye and ENT Hospital of Fudan University (Shanghai, China) between May 2021 and October 2021. The inclusion criteria were as follows: (1) age ≥ 20 years, (2) stable refractive error, (3) preoperative cylinder between −1.50 and −0.50 diopters, and (4) the minimum anterior chamber depth (ACD) was 2.8 mm and the minimum endothelial cell density was 2500 cells/mm2. The exclusion criteria were as follows: (1) concurrent infections of the cornea, (2) concomitant autoimmune diseases, (3) severe dry eye disease, (4) history of other pre-existing ocular diseases, or (5) severe mental disorders, including anxiety and depression.
Seventy eyes from 70 participants were included in the current study. The participants were classified into two groups: SM–CRI and NSM–CRI. Each patient provided written informed consent prior to surgery, after a detailed explanation of the benefits and risks of the study was provided. All subjects were treated in accordance with the tenets of the Declaration of Helsinki. The Ethical Committee of the Eye and ENT Hospital of Fudan University Review Board approved the study protocol.
Surgical Techniques
All surgeries were performed by an experienced surgeon (X.Z.) using the same technique as used in this study. The size of the implanted ICL, V4c, was determined according to the ACD and horizontal white-to-white corneal diameter. Dilating and topical anesthetic agents were administered on the day of surgery. For patients in the SM–CRI group, a clear corneal incision of 3.0 mm was made in the subjective steep meridian of refraction, while for patients in the NSM–CRI group, an incision of the same size was made deviating 15–75° from the steep meridian that would cause oblique torque effects, mostly on the temporal part of the cornea. An ICL V4c was implanted after injection of a viscosurgical substance into the anterior chamber. The ICL was then placed into the posterior chamber. Finally, the viscosurgical substance was replaced with Ringer’s lactate solution. Postoperatively, antibiotics (0.5% levofloxacin; Santen Pharmaceutical, Osaka, Japan) and 1.0% prednisolone acetate (Pred Forte; Allergan, Irvine, CA, USA) were topically administered four times daily for 7 days and pranoprofen (Senju, Osaka, Japan) four times daily for 14 days.
Postoperative Assessment
All patients underwent postoperative ocular examinations at 1 and 6 months after surgery. The following parameters were evaluated: uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, slit-lamp evaluation, intraocular pressure (IOP), corneal topography, and endothelial cell density. Vector analysis was performed for eyes with astigmatic correction using the Alpins method [9, 10]. As suggested by Alpins, the target-induced astigmatism (TIA), surgically induced astigmatism (SIA), difference vector (DV), magnitude of error (ME), angle of error (AE), correction index (CI), and index of success (IOS) were analyzed. Keratometric surgically induced astigmatism (SIAK) was calculated online using the assort vector calculator for ophthalmic refractive surgery techniques (http://www.assort.com/assort-vector-calculator-0).
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics (version 24.0; SPSS Inc., Armonk, NY, USA). The mean ± standard deviation was used for quantitative variables. Differences were considered statistically significant when P < 0.05. The Kolmogorov–Smirnov test for normality was used to confirm data normality. Independent sample t-tests were used to compare clinical variables and astigmatic vector components between the two groups.
Results
All surgeries were uneventful and did not involve any intraoperative complications. The vaults at 6 months postoperatively were 366.71 ± 132.44 µm and 353.97 ± 176.10 µm in the SM–CRI and NSM–CRI groups, respectively. The intraocular pressure in all patients was normal. No complications, such as secondary glaucoma or cataract, occurred in either group throughout the 6 month follow-up period. The preoperative clinical characteristics are presented in Table 1, and no significant difference in preoperative baseline characteristics was observed between the two groups.
Refractive Outcomes
At the postoperative 6 month visit, all participants in both the SM–CRI and NSM–CRI groups achieved an UDVA of 20/20 or better (Figs. 1A and 2A). Compared with the preoperative CDVA, 71% (25/35) and 66% (23/35) of treated eyes in the SM–CRI and NSM–CRI groups, respectively, exhibited a gain of one or more lines in the postoperative UDVA (Figs. 1B and 2B). Similarly, 97.0% (34/35) of treated eyes in the SM–CRI group and 100.0% (35/35) of treated eyes in the NSM–CRI group exhibited unchanged or improved CDVA (Figs. 1C and 2C). Scatter plots of the attempted versus achieved spherical equivalent correction are shown in Figs. 1D and 2D. After surgery, the SE in 86.0% (30/35) of treated eyes in the SM–CRI group and 91% (32/35) of treated eyes in the NSM–CRI group were within ± 0.50 D (Figs. 1E and 2E). The change in manifest SE is shown in Figs. 1F and 2F. As for astigmatism correction, 89.0% (31/35) of the treated eyes in the SM–CRI group and 80.0% (28/35) of the treated eyes in the NSM–CRI group demonstrated postoperative astigmatism within 0.50 D (Figs. 1G and 2G). Scatter plots of the TIA versus SIA vectors and the distribution of AE are shown in Figs. 1H, 2H, 1I, and 2I, respectively.
Visual outcomes of the SM–CRI group at 6 months postoperative. A uncorrected distance visual acuity (UDVA) outcomes. B postoperative UDVA and preoperative corrected distance visual acuity (CDVA). C change in CDVA. D distribution of achieved spherical equivalent outcomes. E spherical equivalent refractive accuracy. F stability of spherical equivalent refraction. G refractive astigmatism. H target induced versus surgically induced astigmatism vectors, and I refractive astigmatism angle of error distribution at 6 months postoperatively. D = diopters
Visual outcomes of the NSM–CRI group at 6 months postoperative. A uncorrected distance visual acuity (UDVA) outcomes. B postoperative UDVA and preoperative corrected distance visual acuity (CDVA). C change in CDVA. D distribution of achieved spherical equivalent outcomes. E spherical equivalent refractive accuracy. F stability of spherical equivalent refraction. G refractive astigmatism. H target induced versus surgically induced astigmatism vectors, and I refractive astigmatism angle of error distribution at 6 months postoperatively. D diopters
Vector Analysis
The TIA of the SM–CRI and NSM–CRI groups were 0.67 ± 0.16 and 0.61 ± 0.21 D, respectively (P = 0.157). At 6 months postoperatively, the mean magnitude of the SIA was 0.56 ± 0.24 and 0.39 ± 0.22 D (P = 0.002), respectively. The DV showed that residual astigmatism in the SM–CRI group was much smaller than that in the NSM–CRI group (P = 0.021). Figure 3 shows single-angle polar plots of the SM–CRI and NSM–CRI groups to display the vector parameter distributions at the 6 month follow-up.
Single-angle polar plots at 6 months postoperative. A surgically induced astigmatism vector of SM–CRI group. B target induced astigmatism vector of SM–CRI group. C difference vector of the SM–CRI group. D correction index of SM-CRI group. E surgically induced astigmatism vector of the NSM–CRI group. F target induced astigmatism vector of the NSM–CRI group. G difference vector of the NSM–CRI group. H correction index of the NSM–CRI group
At 6 months after surgery, the CI was 0.84 ± 0.30 and 0.67 ± 0.35 for the SM–CRI and NSM–CRI groups, respectively, which reached a statistically significant difference (P = 0.013). From the distributions in Figs. 1 and 2, approximately 71% of eyes in the SM–CRI group had an AE within ± 15°, whereas 55% of eyes in the NSM–CRI group were within that range. The absolute mean AE was 10.13 ± 14.57° in the SM–CRI group, compared with 23.88 ± 28.22° in the NSM–CRI group (P = 0.038) (Table 2).
Surgically Induced Astigmatism
At 6 months after surgery, the corneal astigmatism (Astig) showed a significant reduction (−0.33 ± 0.27 D) in the SM–CRI group and a slight increase (0.17 ± 0.30 D) in the NSM–CRI group. The mean magnitude of the SIAK was 0.53 ± 0.33 D, and the flattening effect was 0.41 ± 0.27 D in the SM–CRI group. In the NSM–CRI group, the mean magnitude of the SIAK was 0.38 ± 0.19 D, and the flattening effect was 0.30 ± 0.21 D. The mean SIAK values were 0.38 at 8° in the SM–CRI group and 0.20 at 96° in the NSM–CRI group (Table 3).
Discussion
SM–CRI, as an economical and simple surgical method, has been widely used to correct mild and moderate astigmatism in cataract surgery [11, 12]. In the ICL study, we made an incision according to the steep meridian of the manifest refraction to correct the preoperative astigmatism. SM–CRI in ICL surgery can avoid misalignment of the TICL, thus reducing the risk and cost of complications of ICL exchange. The results of our prospective randomized study indicate that both the SM–CRI and NSM–CRI groups achieved good refractive outcomes in terms of predictability, efficacy, and safety. All surgeries were uneventful and no obvious intraoperative or postoperative complications were observed during the follow-up period. Moreover, UDVA and CDVA were significantly increased after surgery. However, the postoperative refractive astigmatism in the SM–CRI group was significantly less than that in the NSM–CRI group (DV: 0.25 versus 0.39 D).
Six months after surgery, we found that the CI of both groups was less than 1, suggesting undercorrection of astigmatism. However, since most young patients have with-the-rule (WTR) astigmatism, slight undercorrection is acceptable. The CI of the SM–CRI group was 0.84, while that of the NSM–CRI group was only 0.67, suggesting that the SM–CRI has significant advantages in astigmatism correction. In addition, we also found that SM–CRI had a smaller effect on the axis of astigmatism (AE: 23.88° versus 10.13°) than NSM–CRI, which is also an advantage of SM–CRI over other incision sites. Therefore, the SM–CRI can reduce the cylindrical diopter of the ICL. As for the stability of astigmatism correction, no statistically significant changes were found between the two groups during the follow-up period of 1 and 6 months after surgery. Therefore, we can conclude that SM–CRI has better benefits for ICL with low astigmatism.
In our study, SIA was 0.53 D in the SM–CRI group and 0.38 D in the NSM–CRI group. In a comparative study of incision sites for cataracts, He et al. reported that SIA of the steep-meridian incision and non-steep-meridian incision were 0.50 and 0.54 D, respectively, with no significant difference [8]. Borasio observed a significant reduction in SIA through a temporal incision (0.34 D) compared with a steep meridian incision (0.63 D) in cases of mild-to-moderate astigmatism [13, 14]. In addition, since young patients with WTR were the main subjects of this study, the superior incision was mainly used for SM–CRI, while the temporal incision was mainly used for NSM–CRI. The study by Kamiya on low-to-moderate astigmatism showed that SIA of superior and temporal incisions were 0.57 and 0.48 D, respectively, which was consistent with our study findings [2]. Previous studies have assumed that making an incision at the steep meridian can flatten it, while making an incision at the flat meridian can steepen it, thus theoretically it may correct some astigmatism without changing the axis of the astigmatism [13]. In our study, corneal astigmatism in the SM–CRI group was significantly alleviated 6 months after surgery, while that in the NSM–CRI group was slightly increased, which was related to the corneal relaxing effect of the incision site. The vector analysis results of SIA also indicated that there was a tendency for corneal flattening towards the incision site, with the introduction of against-the-rule (ATR) (0.38 at 8°) and WTR(0.20 at 96°) for the superior and temporal incisions, respectively, which is consistent with the results of Tejedor [15]. To some extent, this conclusion explains why the SM–CRI group had better astigmatism correction efficacy as a non-toric ICL was included in this study.
Notably, although corneal astigmatism in the NSM–CRI group increased slightly, the manifest astigmatism decreased significantly, which is consistent with the conclusion of Kamiya’s previous study [16]. In their study, the horizontal incision caused corneal astigmatism to increase from 1.16 D at 90.5° to 1.45 D at 90.2°, while the manifest astigmatism decreased from 0.93 D at 93.2° to 0.72 D at 87.4°. They believed that the role of lens accommodation in lens astigmatism may lead to a difference between the cornea and manifest astigmatism after ICL implantation. In addition, ICLs, such as corneal contact lenses, appear to compensate for small amounts of lenticular astigmatism, thus contributing to the reduction in manifest astigmatism.
Limbal relaxing incision (LRI) is also used for astigmatism correction. Riaz et al. demonstrated that LRI during cataract surgery achieved an effective and sustained reduction of both refractive and keratometric astigmatism, regardless of the meridian or magnitude of astigmatism; this correction persisted for at least 1 year postoperatively [17]. Compared with on-axis incision, the level of astigmatism reduction achieved at the intended meridian was significantly more favorable with the LRI technique [18]. Li et al. also reported that LRI effectively reduces corneal astigmatism during ICL surgery [19]. Although recent research has demonstrated the usefulness of LRI in reducing astigmatism, this technique is an additional method that is not a part of the ICL surgery procedure.
There are still some limitations to this study, including the small sample size and the lack of randomization. A previous study on cataract surgery indicated that the flattening effect of temporal incisions, but not superior incisions, performed on the preoperative corneal steep meridian differed between the right and left eyes [20]. Additionally, we did not observe the visual quality of the entire eye. Future studies should include a larger number of cases in randomized control trials and assess the ipsilateral eye.
Conclusions
SM–CRI can alleviate corneal astigmatism and decrease the cylindrical diopter in the ICL, thus improving postoperative visual quality compared with that achieved by NSM–CRI. Therefore, SM–CRI might be better to address low-to-moderate astigmatism during ICL implantation.
References
Monteiro T, Pinto C, Franqueira N, Faria-Correia F, Mendes J, Alfonso Sanchez J, Vaz F. Efficacy and safety after toric posterior chamber implantable collamer lens and toric iris-fixated foldable phakic intraocular lens for myopic astigmatism. J Refract Surg. 2022;38(6):339–47.
Kamiya K, Ando W, Takahashi M, Shoji N. Comparison of magnitude and summated vector mean of surgically induced astigmatism vector according to incision site after phakic intraocular lens implantation. Eye Vis. 2021;8(1):32.
Wei R, Li M, Niu L, Aruma A, Miao H, Shen Y, Yao P, Wang X, Zhang H, Zhou X. Comparison of visual outcomes after non-toric and toric implantable collamer lens V4c for myopia and astigmatism. Acta Ophthalmol. 2021;99(5):511–8.
Alonso-Juarez E, Velazquez-Villoria D. Low diopter phakic implantable collamer lens: refractive and visual outcomes in low myopia and myopic astigmatism. Clin Ophthalmol. 2022;16:2969–77.
Zhu M, Zhu L, Zhu Q, Xu C, Yu P, Xiao H, Wang Y, Yuan Y. Clinical effect and rotational stability of TICL in the treatment of myopic astigmatism. J Ophthalmol. 2020;2020:3095302.
Yoon YC, Ha M, Whang WJ. Comparison of surgically induced astigmatism between anterior and total cornea in 2.2 mm steep meridian incision cataract surgery. BMC Ophthalmol. 2021;21(1):373.
Li PP, Huang YM, Cai Q, Huang LL, Song Y, Guan HJ. Effects of steep-axis incision on corneal curvature in one-handed phacoemulsification. Int J Ophthalmol. 2019;12(8):1277–82.
He W, Zhu X, Du Y, Yang J, Lu Y. Clinical efficacy of implantation of toric intraocular lenses with different incision positions: a comparative study of steep-axis incision and non-steep-axis incision. BMC Ophthalmol. 2017;17(1):132.
Hernandez R, Almenara C, Soriano D, Idoipe M, Larrosa JM, Pablo LE, Garcia-Martin E. Toric intraocular lens implantation vs femtosecond laser-assisted arcuate keratotomy for correction of moderate astigmatism in cataract surgery. J Cataract Refract Surg. 2022;48(8):887–93.
Kose B. Comparison of refractive and visual outcomes between image-guided system-assisted small-incision lenticule extraction and wavefront-optimized FS-LASIK in treatment of high astigmatism. J Cataract Refract Surg. 2022;48(7):765–70.
Patil MS, Nikose AS, Bharti S. Visual outcome and refractive status with monofocal toric intraocular lens implantation to correct astigmatism during cataract surgery. Indian J Ophthalmol. 2020;68(12):3016–9.
Ren Y, Fang X, Fang A, Wang L, Jhanji V, Gong X. Phacoemulsification With 3.0 and 2.0 mm opposite clear corneal incisions for correction of corneal astigmatism. Cornea. 2019;38(9):1105–10.
Borasio E, Mehta JS, Maurino V. Torque and flattening effects of clear corneal temporal and on-axis incisions for phacoemulsification. J Cataract Refract Surg. 2006;32(12):2030–8.
Borasio E, Mehta JS, Maurino V. Surgically induced astigmatism after phacoemulsification in eyes with mild to moderate corneal astigmatism: temporal versus on-axis clear corneal incisions. J Cataract Refract Surg. 2006;32(4):565–72.
Tejedor J, Murube J. Choosing the location of corneal incision based on preexisting astigmatism in phacoemulsification. Am J Ophthalmol. 2005;139(5):767–76.
Kamiya K, Shimizu K, Aizawa D, Igarashi A, Komatsu M. Surgically induced astigmatism after posterior chamber phakic intraocular lens implantation. Br J Ophthalmol. 2009;93(12):1648–51.
Riaz KM, Wang L, Williams B, Dvorak JD, Kloek CE, Farooq AV, Koch DD. Refractive and keratometric outcomes of supervised novice surgeon-performed limbal relaxing incisions: 1-year results. J Cataract Refract Surg. 2021;47(10):1319–26.
Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M. Queen Elizabeth astigmatism study G: limbal relaxing incisions versus on-axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31(12):2261–5.
Li Z, Han Y, Hu B, Du H, Hao G, Chen X. Effect of Limbal relaxing incisions during implantable collamer lens surgery. BMC Ophthalmol. 2017;17(1):63.
Alpins N, Ong JK, Stamatelatos G. Asymmetric corneal flattening effect after small incision cataract surgery. J Refract Surg. 2016;32(9):598–603.
Acknowledgements
We thank all study participants for their involvement in the study.
Funding
The study and its publication, including the journal’s Rapid Service Fee, were partly supported by the National Natural Science Foundation of China (Grant No. 81770955), Joint Research Project of New Frontier Technology in Municipal Hospitals (SHDC12018103), Shanghai Science and Technology Project (Grant No. 20410710100), Clinical Research Plan of SHDC (SHDC2020CR1043B), Project of Shanghai Xuhui District Science and Technology (2020–015) (XHLHGG202104), and Shanghai Engineering Research Center of Laser and Autostereoscopic 3D for Vision Care (20DZ2255000).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Shengtao Liu had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: Shengtao Liu and Feng Lin. Drafting of the manuscript: Jingying Liu and Chiwen Cheng. Critical revision of the manuscript for important intellectual content: Shengtao Liu and Xingtao Zhou. Statistical analysis: All authors. Obtained funding: Shengtao Liu and Xingtao Zhou. Administrative, technical, or material support: Shengtao Liu and Lanhui Yu. Supervision: Ti Wang and Xingtao Zhou.
Disclosures
Shengtao Liu, Jingying Liu, Feng Lin, Lanhui Yu, Chiwen Cheng, Ti Wang and Xingtao Zhou have nothing to disclose.
Compliance with Ethics Guidelines
The study followed the requirements of medical ethics and all the patients provided written informed consent before surgery. All subjects were treated in accordance with the tenets of the Declaration of Helsinki. The Ethical Committee of the Eye and ENT Hospital of Fudan University Review Board approved the study protocol.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, S., Liu, J., Lin, F. et al. Efficacy Comparison Between Steep-Meridian Incision and Non-Steep-Meridian Incision in Implantable Collamer Lens Surgery with Low-to-Moderate Astigmatism. Ophthalmol Ther 12, 1711–1722 (2023). https://doi.org/10.1007/s40123-023-00704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00704-1