Abstract
Introduction
This study analyzed the visual outcome following cataract surgery with toric intraocular lenses (IOLs) in patients older than 80 years with corneal astigmatism.
Methods
A total of 159 patients (159 eyes) older than 80 years with corneal astigmatism (≥ 0.75 D) were included. Fifty-three eyes received Acrysof IQ® toric IOLs (SN6AT2–5), while the others received non-toric IOLs: 51 eyes received Acrysof IQ® IOLs (SN60WF) and 55 eyes received A1-UV IOLs. The uncorrected distance visual acuity, corrected distance visual acuity, and refraction (spherical equivalent, refractive cylinder) were assessed at 3 months postoperatively. The prediction error of refractive outcome and percentages of eyes within ± 0.50 D and ± 1.00 D in the toric IOL group obtained using five toric IOL formulas (Barrett predicted posterior corneal astigmatism (PCA), Barrett measured PCA, Kane, EVO 2.0 and Næser–Savini) were compared.
Results
At 3 months postoperatively, the average uncorrected distance visual acuity was better in the toric IOL group than the non-toric IOL group (p < 0.001). The mean residual refractive cylinder was lower in the toric IOL group than the non-toric IOL group (p < 0.001). The Næser–Savini formula achieved the lowest mean absolute error (0.39 D) and had the highest percentages of eyes within an absolute error of 0.50 D and 1.00 D (72% and 98%) compared to the other formulas.
Conclusion
The results demonstrate the efficacy of toric IOL implantation in patients older than 80 years with corneal astigmatism and provide strong evidence for cataract surgeons to encourage such patients to choose toric IOLs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Previous studies have demonstrated the effectiveness of toric intraocular lenses (IOLs). However, due to a number of factors, toric IOL implantation may not be the first choice of treatment for many older adult patients. |
What was learned from the study? |
The outcomes of toric IOLs are better than those of non-toric IOLs in patients ≥ 80 years old with cataracts and corneal astigmatism. Cataract surgeons should consider recommending toric IOLs for patients who are ≥ 80 years old with regular corneal astigmatism. |
Introduction
The Chinese population is currently undergoing societal aging, and the prevalence of cataracts increases markedly with increasing age [1]. In addition, increased age is associated with an increased risk of complications and bad surgical outcomes [2]. Cataracts are the most important forms of reversible visual impairment. It is estimated that significant cataracts are present in about half of all subjects in their seventies and all subjects in their nineties [3]. Reduced vision is associated with decreased ability to perform activities of daily living, decreased social functioning, and reduced life expectancy among older adults [4,5,6,7].
Escalating patient expectations and greater recognition of visual quality after cataract extraction have caused a shift from cataract surgery to refractive surgery. Toric intraocular lenses (IOLs) have been designed to replace cataract lenses, reduce postoperative astigmatism, and reduce or eliminate the need for distance-vision spectacles or additional surgery. However, due to traditional views, neglect of the quest for higher visual quality, financial constraints, and so on, toric IOL implantation is not the first choice of treatment for many older adult patients. Some characteristics of patients older than 80 years are that (1) the corneal astigmatism is commonly > ~ 1.1 D [8] and (2) some of the patients are more reluctant to wear glasses due to poor memory [9] and inconvenience [10].
The primary aim of the present study was to analyze the clinical outcomes following cataract surgery with toric IOL implantation in patients older than 80 years and to assess the refractive predictions of modern toric IOL calculation formulas for this specific age group.
Methods
Patients
This retrospective study was performed at the Eye Hospital of Wenzhou Medical University, Hangzhou, China. All patients underwent phacoemulsification and IOL implantation from 2020 to 2022. The inclusion criteria were age over 80 years and corneal astigmatism (≥ 0.75 D). The toric IOL group received Acrysof IQ® toric IOLs (SN6ATx, Alcon Inc.), while the non-toric IOL group (comparison group) received Acrysof IQ® IOLs (SN60WF Alcon Inc.) or A1-UV IOLs (Eyebright, China). When both eyes of a patient were eligible, the right eye was defined as the number 0 and the left eye as 1. An online random number generator (https://www.iikx.com/tool/radom.html) was then used to determine which eye to include. The exclusion criteria were congenital ocular abnormalities, use of ocular medications that may affect vision, previous retinal disease, glaucoma, previous corneal disease, history of eye trauma, previous cerebrovascular accidents, peri- or postoperative complications, degenerative eye disorders, moderate and severe dry eye disease, and previous refractive surgery.
The Institutional Review Board of Wenzhou Medical University approved the study and waived the requirement for informed consent because of the retrospective nature of the study. Anonymized patient data were used for the analysis, and the study adhered to the principles of the Declaration of Helsinki.
Preoperative Preparation
Patients with a mild degree [11, 12] of dry eye disease and severe cataracts were enrolled in the study. Dry eye disease of patients with moderate and severe meibomian gland dysfunction (MGD) was ruled out after a series of ocular surface assessments. We firstly collected the history, assessed the eyelid margin under a slit lamp, and evaluated the meibomian gland using meibography via a noncontact meibography system (Kerotography 5 M, Oculus, Wetzlar, Germany). Furthermore, the dry eye questionnaire scale (SPEED) was applied to assess the condition of dry eye disease for each patient.
All other ocular biometry measurements were performed using a Zeiss IOLMaster 500 or 700 (Carl Zeiss, Meditec). A Pentacam (Oculus Optikger EUROAL ATE GmbH) was used to rule out irregular corneal astigmatism and evaluate the curvature of the posterior corneal surface. IOL spherical power was calculated using the Barrett Universal II formula. The cylinder power and target IOL axis were calculated using the Alcon online calculator based on the IOLMaster data (http://www.myalcon-toriccalc.com/). A surgically induced astigmatism of 0.22 D was assumed to fill the calculator.
After topical anesthesia with propantheline (Alcon-Couvreur), each patient was instructed to sit upright, keep their head carefully aligned, and fix their eyesight straight on the distant target with the nonsurgical eye. The light from the slit lamp was adjusted to a sharp line, turned to align with the target axis and the 135° location of the main corneal incision separately, and centered on the corneal apex to ensure that it passed through the middle of the cornea. The target axis position was marked as a sharp line by scratching the corneal epithelium at the limbus with a needle; the main corneal incision was marked in the same way. Thereafter, the scratch marks were stained using a surgical marking pen. The comparison group were examined preoperatively as described above except for the preoperative marking step.
Surgical Procedures
The same experienced surgeon performed all surgeries. Surgery was performed through a 2.2-mm clear corneal incision at 135° of the corneal limbus. A 5.0- to 5.5-mm continuous curvilinear capsulorhexis was then made, followed by hydrodissection, phacoemulsification, and posterior capsule polishing. The toric IOL was implanted with a Monarch III delivery system and rotated into a position approximately 10–20° short of the intended axis. Finally, the IOL was aligned to the correct position after removing the residual viscoelastic device. The IOL axis was double-checked after the incision was hydrated and the anterior chamber was reinflated. The comparison group was operated on in the same way except for the axial alignment.
Postoperative Examination and Calculation
The following visual outcomes were analyzed 3 months after surgery for all patients: postoperative refractive spherical equivalent, refractive cylinder, uncorrected distance visual acuity (UDVA), and corrected distance visual acuity (CDVA). Before any further analysis, all left eye refractive data (pre- and postoperative) were converted; as a result, the new axis of the left eye was equal to 180° minus the original axis [13, 14]. The decimal visual acuity (VA) data were converted to logMAR for statistical analysis and the calculation of the average VA using a standard conversion formula.
The power data are represented by the mean sphere M and two crossed cylinders J0 and J45 [15]. The magnitude of the astigmatic power vector (APV) represents the magnitude of the astigmatic error. The conversion process is as follows:
Here, S is the spherocylindrical sphere power, C is the signed cylinder power, and α is the cylinder axis. The above data results were used for statistical analysis.
Five toric IOL formulas [Barrett predicted posterior corneal astigmatism (PCA), Barrett measured PCA, Kane, EVO 2.0, and Næser–Savini] were employed using each patient’s preoperative ocular biometry measurement data (real world analysis [16]), which were entered into the respective calculators (Barrett [predicted or measured PCA]: https://ascrs.org/tools/barrett-toric-calculator; Kane: https://www.iolformula.com/agreement/; EVO 2.0: https://www.evoiolcalculator.com/toric.aspx; Næser–Savini: https://www.sedesoi.com/toric-2021/). We used the vector analysis [17]. The actual postoperative refractive astigmatism was calculated by vertexing the postoperative manifest refractive astigmatism to the corneal plane. The prediction error (PE) was calculated as the vector difference between the actual and predicted postoperative refractive astigmatism. The median absolute error (MedAE), mean absolute error (MAE), vector calculated centroid error [mean centroid (diopters @ degree-s) ± SD], and percentages of eyes with a postoperative absolute error (AE) within 0.50 D and 1.00 D were calculated.
Statistical Analysis
The sample size was calculated according to the main indicators [mean UDVA (logMAR); postoperative mean refractive cylinder (D); APV (D)], and the results showed that the total number of samples needed was more than 90 (each group was 30). Graphs for intraocular lens-based refractive surgery were drawn according to the standard requirement [18]. Statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). The chi-squared test was used to compare both the gender composition and the right to left eye ratio among the three groups. The normality of the distributions was evaluated using the Shapiro–Wilk test. For comparative statistics, if the data were normally distributed, a one-way ANOVA test was selected; otherwise, the Kruskal–Wallis test was used. Values were expressed as the mean ± standard deviation. If the Kruskal–Wallis test was used, the median and interquartile range (P1;P3) were also presented to provide a statistical description. The Hotelling test was used to perform multivariate statistical analysis of the centroid errors [16]. Statistical significance was set at p < 0.05.
Results
Patient Demographics
The demographics and clinical features of the 159 patients (159 eyes) are shown in Table 1. When both eyes of a patient were eligible, one eye was selected at random for analysis. Among them, 53 eyes received toric IOLs, 51 eyes received IQ IOLs, and 55 eyes received A1-UV IOLs. Figure 1 summarizes the patient selection process. The average age in the toric IOL, IQ IOL, and A1-UV IOL groups was 83.98 ± 2.98 years, 84.08 ± 3.11 years, and 83.62 ± 2.99 years, respectively. The IOL spherical power, anterior chamber depth (ACD), axial length (AL), average keratometry, and corneal astigmatism did not significantly differ between the three groups. The numbers and types of IOLs inserted are shown in Table 1.
Postoperative Refraction and Visual Acuity
Table 2 shows the refraction and VA outcomes. The mean residual refractive cylinder was 0.58 ± 0.36 D in the toric IOL group, 1.10 ± 0.53 D in the IQ IOL group (p < 0.001), and 1.35 ± 0.90 D in the A1-UV IOL group (p < 0.001). The APV was 0.29 ± 0.18 D in the toric IOL group, 0.55 ± 0.26 D in the IQ IOL group (p < 0.001), and 0.68 ± 0.45 D in the A1-UV IOL group (p < 0.001). The toric IOL group showed a significantly better mean UDVA (0.15 ± 0.10 logMAR; p < 0.001) compared with the non-toric group. No significant difference in the CDVA was found between the toric IOL group and the comparison groups. Figure 2 shows a plot of J0 versus J45 for the postoperative refractions in both the toric and the non-toric groups.
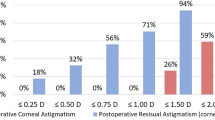
Figure 3 shows standard graphs for reporting the refractive outcomes for IOL-based procedures in a cataract population. A Snellen VA of 20/40 or better was achieved by 98% (n = 52) of the patients in the toric IOL group (Fig. 3A). Overall, 49% (n = 26) of the eyes with toric IOLs had a UDVA that was the same as the CDVA (Fig. 3B). A spherical equivalent refraction of – 0.5 to – 0.14 was achieved in 42% (n = 19), 31% (n = 16), and 31% (n = 17) of the toric IOL, IQ IOL, and A1-UV IOL groups, respectively (Fig. 3C). A postoperative refractive cylinder of less than 1.00 D was achieved in 94% (n = 51), 57% (n = 29), and 44% (n = 24) of the eyes in the toric IOL, IQ IOL, and A1-UV IOL groups, respectively (Fig. 3D).
Standard graphs for reporting refractive outcomes of intraocular lens-based procedures in a cataract population. A Uncorrected distance visual acuity. B Uncorrected distance visual acuity versus corrected distance visual acuity. C Spherical equivalent refraction accuracy. D Postoperative refractive cylinder. CDVA corrected distance visual acuity, UDVA uncorrected distance visual acuity
Accuracies of the Five Toric IOL Formulas
Table 3 shows the refractive astigmatism error results for the five toric IOL formulas. The Næser–Savini formula achieved the lowest MAE (0.39 D), though no statistically significant difference was found between the five formulas (p = 0.435). Using the vector algorithm, the centroid of the PE varied significantly from 0 for the Kane formula (p < 0.001) and was not statistically significantly different from 0 for the other formulas (p > 0.05). Figure 4 shows the double-angle plots of the PE for each toric IOL formula.
Discussion
The present study demonstrated that toric IOL implantation gave better visual outcomes than non-toric lens implantation in patients older than 80 years with corneal astigmatism (≥ 0.75D). The availability of medical insurance and the additional cost of a toric IOL may be a burden for some frugal or low-income patients, especially in the older adult population in China. However, a VA improvement in very old patients translates into improved quality of life and postural stability and a reduction in fall-related fractures, which would benefit patients, families, society, and the nation.
The comparison group in the present study included both IQ and A1-UV IOLs rather than solely IQ IOLs because the A1-UV IOL has become increasingly popular due to its good clinical outcomes and suitable price in China. A previous study [19] showed that a comparison between the A1-UV IOL and SN60WF IOL can minimize the potential impact of material as these lenses use the same hydrophobic acrylic materials and have similar profiles. Therefore, for some aspects, the A1-UV IOL can be used as a control IOL in clinical studies.
In patients with a cataract alone, significant improvements in UDVA are often reported when using toric IOLs compared with non-toric IOLs [20, 21]. Dan et al. [18] noted that the preoperative CDVA does not help when assessing the refractive outcomes in a population of patients with cataracts because removing the lens typically achieves a significant improvement in VA, independent of the refractive correction. Thus, the UDVA is reported as the best measurement of the VA. In the present study, compared with non-toric IOLs, the use of toric IOLs significantly improved the UDVA. These results are consistent with previous reports [20, 21]. Although there were differences in preoperative corneal astigmatism between the three groups, the mean residual refractive cylinder of the toric IOL group was significantly less than that of the comparison group postoperatively. Minimizing refractive astigmatism after cataract surgery can significantly improve a patient's vision [22]. The present study found a significant improvement in postoperative refractive astigmatism and UDVA after toric IOL implantation compared with non-toric IOL implantation in patients older than 80 years with corneal astigmatism. This, in turn, increases the chances of postoperative spectacle independence and decreases the possibility of complications caused by additional correcting astigmatism surgery. Therefore, age should not be a limiting factor in the quest for better visual quality.
Elderly patients are of concern because their eyes change as they get older. Some studies have shown that older age is associated with structural changes to collagen that impact scleral fiber alignment, matrix stiffness [23], and the stretching qualities of the anterior capsule and lens zonule. This changes the elastic properties over time [24, 25]. Moreover, age has been shown to affect the morphology of Schlemm's canal and the trabecular meshwork [26]. ACD changes have also been reported in the older adult population [27]: the AL is significantly shortened and the ACD becomes shallower with increasing age. Increased age is associated with a flatter corneal curvature, although the correlation is weak. Accordingly, the AL, ACD, and corneal curvature at baseline may contribute to the degree of prediction error, possibly through age-related changes in these parameters [28]. In addition, dry eye, mental status, and systemic comorbidities may hamper the ability of older adults to cooperate with meticulous manifest refraction or fixation to achieve technically decent biometry, which affects the refractive outcome and accuracy of IOL calculation formulas [29].
No studies have attempted to assess the refractive predictions of modern toric IOL calculation formulas in the population older than 80 years. Therefore, the present study aimed to compare the accuracies of toric IOL formulas (Barrett [predicted and measured PCA], Kane, EVO 2.0, and Næser–Savini) in patients older than 80 years. The Barrett toric IOL formula uses the Barrett Universal 2 formula to calculate the effective lens position (ELP), and uses a PCA prediction algorithm or actual measurements to predict the actual postoperative refractive astigmatism. The Kane toric IOL formula uses the Kane formula to calculate the ELP before using an advanced algorithm incorporating regression, theoretical optics, and artificial intelligence techniques to calculate the total corneal astigmatism [30, 31]. The EVO 2.0 toric IOL formula uses the EVO 2.0 formula to calculate the ELP, and uses a theoretical posterior corneal astigmatism prediction to calculate the total corneal power. The Næser–Savini formula uses equations to modify the measured anterior corneal astigmatism to better represent the overall corneal astigmatism [32]. In the current study, there was no statistically significant difference among the five formulas. Therefore, we consider that the five formulas were consistent in their effectivenesses for the present sample. It is also possible that the present sample size was too small to detect the most accurate formula. A study with a larger sample size is required to compare the accuracies of toric IOL formulas.
The present study had several limitations. First, the sample size of patients older than 80 years with toric IOL implantation was comparatively small. Second, VA was the only objective measure of vision used. Moreover, the relatively short follow-up for VA limits the estimation of long-term results. Measuring contrast sensitivity may add helpful information.
Conclusion
In conclusion, visual outcomes were better in patients 80 years and older with corneal astigmatism who underwent implantation of toric IOLs than in those implanted with non-toric IOLs. The accuracies of five toric formulas were excellent and consistent in the patients. In future clinical applications, cataract surgeons should consider recommending toric IOLs for patients 80 years and older with corneal astigmatism.
References
Nussinovitch H, Tsumi E, Tuuminen R, Malyugin B, Lior Y, Naidorf Rosenblatt H, Boyko M, Achiron A, Knyazer B. Cataract surgery in very old patients: a case-control study. J Clin Med. 2021;10(20):4658. https://doi.org/10.3390/jcm10204658.
Toyama T, Ueta T, Yoshitani M, Sakata R, Numaga J. Visual acuity improvement after phacoemulsification cataract surgery in patients aged ≥90 years. BMC Ophthalmol. 2018;18(1):280. https://doi.org/10.1186/s12886-018-0950-8.
Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, Riva-Cambrin J. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9(4):e025091. https://doi.org/10.1136/bmjopen-2018-025091.
Şahlı E, İdil ŞA. Comparison of quality of life questionnaires in patients with low vision. Turk J Ophthalmol. 2021;51(2):83–8. https://doi.org/10.4274/tjo.galenos.2020.99975.
Umfress AC, Brantley MA. Eye care disparities and health-related consequences in elderly patients with age-related eye disease. Semin Ophthalmol. 2016;31(4):432–8. https://doi.org/10.3109/08820538.2016.1154171.
Clarke EL, Evans JR, Smeeth L. Community screening for visual impairment in older people. Cochrane Database Syst Rev. 2018;2:CD001054. https://doi.org/10.1002/14651858.CD001054.pub3
Ehrlich JR, Ramke J, Macleod D, Burn H, Lee CN, Zhang JH, Waldock W, Swenor BK, Gordon I, Congdon N, Burton M, Evans JR. Association between vision impairment and mortality: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(4):e418–30. https://doi.org/10.1016/S2214-109X(20)30549-0.
Pang YL, Yuan L, Cao XG, Hou XR, Bao YZ. Characteristics and analysis of corneal astigmatism in age-related cataract patients over 50 years old. Zhonghua Yan Ke Za Zhi. 2020;56(5):349–55. https://doi.org/10.3760/cma.j.cn112142-20190618-00323.
Rotenberg S, Sternberg S, Maeir A. Where did I put my glasses? The lived experience of older adults seeking medical help for perceived memory problems. Disabil Rehabil. 2020;42(25):3606–13. https://doi.org/10.1080/09638288.2019.1602849.
Otte B, Woodward MA, Ehrlich JR, Stagg BC. Self-reported eyeglass use by US medicare beneficiaries aged 65 years or older. JAMA Ophthalmol. 2018;136(9):1047–50. https://doi.org/10.1001/jamaophthalmol.2018.2524.
Chinese Branch of the Asian Dry Eye Society, Ocular Surface and Tear Film Diseases Group of Ophthalmology Committee of Cross-Straits Medicine Exchange Association, Ocular Surface and Dry Eye Group of Chinese Ophthalmologist Association. Expert consensus on dry eye in China: dry eye related to eye surgery. Zhonghua Yan Ke Za Zhi. 2021;57(8):564–572. https://doi.org/10.3760/cma.j.cn112142-20210429-00196
Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672–7. https://doi.org/10.1016/j.jcrs.2015.01.016.
Eydelman MB, Drum B, Holladay J, Hilmantel G, Kezirian G, Durrie D, Stulting RD, Sanders D, Wong B. Standardized analyses of correction of astigmatism by laser systems that reshape the cornea. J Refract Surg. 2006;22(1):81–95. https://doi.org/10.3928/1081-597X-20060101-16.
Kawahara A, Takayanagi Y. Comparison of refractive and keratometric astigmatism after microincision cataract surgery. J Cataract Refract Surg. 2017;43(8):1050–3. https://doi.org/10.1016/j.jcrs.2017.05.033.
Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74(6):367–75. https://doi.org/10.1097/00006324-199706000-00019.
Kane JX, Connell B. A comparison of the accuracy of 6 modern toric intraocular lens formulas. Ophthalmology. 2020;127(11):1472–86.
Alpins N. Analysis of aggregate surgically induced refractive change, prediction error, and intraocular astigmatism. J Refract Surg. 2001;17(6):705–7.
Reinstein DZ, Archer TJ, Srinivasan S, Mamalis N, Kohnen T, Dupps WJ, Randleman JB. Standard for reporting refractive outcomes of intraocular lens-based refractive surgery. J Cataract Refract Surg. 2017;43(4):435–9. https://doi.org/10.1016/j.jcrs.2017.04.005.
Liao X, Li JY, Tan QQ, Tian J, Lin J, Lan CJ. Comparison of visual quality after implantation of A1-UV and SN60WF aspheric intraocular lens. Int J Ophthalmol. 2020;13(11):1727–32. https://doi.org/10.18240/ijo.2020.11.07.
Statham M, Apel A, Stephensen D. Comparison of the AcrySof SA60 spherical intraocular lens and the AcrySof Toric SN60T3 intraocular lens outcomes in patients with low amounts of corneal astigmatism. Clin Exp Ophthalmol. 2009;37(8):775–9. https://doi.org/10.1111/j.1442-9071.2009.02154.x.
Lane SS, Ernest P, Miller KM, Hileman KS, Harris B, Waycaster CR. Comparison of clinical and patient-reported outcomes with bilateral AcrySof toric or spherical control intraocular lenses. J Refract Surg. 2009;25(10):899–901. https://doi.org/10.3928/1081597X-20090617-05.
Sun XY, Vicary D, Montgomery P, Griffiths M. Toric intraocular lenses for correcting astigmatism in 130 eyes. Ophthalmology. 2000;107(9):1776–1781; discussion 1781–1782. https://doi.org/10.1016/s0161-6420(00)00266-9.
Coudrillier B, Pijanka J, Jefferys J, Sorensen T, Quigley HA, Boote C, Nguyen TD. Effects of age and diabetes on scleral stiffness. J Biomech Eng. 2015. https://doi.org/10.1115/1.4029986.
Yaguchi S, Bissen-Miyajima H, Ota Y, Oki S, Minami K. Efficacy of femtosecond laser-assisted capsulotomy: experimental evaluation using the zonular dehiscence model. Transl Vis Sci Technol. 2020;9(13):7. https://doi.org/10.1167/tvst.9.13.7.
Michael R, Mikielewicz M, Gordillo C, Montenegro GA, Pinilla Cortés L, Barraquer RI. Elastic properties of human lens zonules as a function of age in presbyopes. Invest Ophthalmol Vis Sci. 2012;53(10):6109–14. https://doi.org/10.1167/iovs.11-8702.
Chen Z, Sun J, Li M, Liu S, Chen L, Jing S, Cai Z, Xiang Y, Song Y, Zhang H, Wang J. Effect of age on the morphologies of the human Schlemm’s canal and trabecular meshwork measured with swept-source optical coherence tomography. Eye (Lond). 2018;32(10):1621–8. https://doi.org/10.1038/s41433-018-0148-6.
Hashemi H, Yekta A, Khodamoradi F, Aghamirsalim M, Asharlous A, Assadpour M, Khabazkhoob M. Anterior chamber indices in a population-based study using the Pentacam. Int Ophthalmol. 2019;39(9):2033–40. https://doi.org/10.1007/s10792-018-1037-5.
Hayashi K, Ogawa S, Yoshida M, Yoshimura K. Influence of patient age on intraocular lens power prediction error. Am J Ophthalmol. 2016;170:232–7. https://doi.org/10.1016/j.ajo.2016.08.016.
Sella R, Chou L, Schuster AK, Gali HE, Weinreb RN, Afshari NA. Accuracy of IOL power calculations in the very elderly. Eye (Lond). 2020;34(10):1848–55. https://doi.org/10.1038/s41433-019-0752-0.
Connell BJ, Kane JX. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4(1): e000251. https://doi.org/10.1136/bmjophth-2018-000251.
Melles RB, Kane JX, Olsen T, Chang WJ. Update on intraocular lens calculation formulas. Ophthalmology. 2019;126(9):1334–5. https://doi.org/10.1016/j.ophtha.2019.04.011.
Savini G, Næser K, Schiano-Lomoriello D, Ducoli P. Optimized keratometry and total corneal astigmatism for toric intraocular lens calculation. J Cataract Refract Surg. 2017;43(9):1140–8. https://doi.org/10.1016/j.jcrs.2017.06.040.
Acknowledgements
Funding
No funding or sponsorship was received for this study or the publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
We would like to thank Editage (www.editage.com) for English language editing.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Pingjun Chang and Yalan Wang conceived and designed the presented study. Xicong Lou, Shuyi Qian, Bin Hu, and Fuman Yang performed the data collection. Yalan Wang performed the analysis and wrote the manuscript. Yune Zhao provided a critical review of the manuscript.
Disclosures
Yalan Wang, Fuman Yang, Xicong Lou, Shuyi Qian, Bin Hu, Yune Zhao, and Pingjun Chang have nothing to disclose.
Compliance with Ethics Guidelines
Approval was obtained from the Institutional Review Board of the Eye Hospital of Wenzhou Medical University (number: 2021-036-K-29-01). The requirement for informed consent was waived because of the retrospective nature of the study. Anonymized patient data were used for the analysis, and the study adhered to the principles of the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Y., Yang, F., Lou, X. et al. Efficacy of Toric Intraocular Lens Implantation in Patients Older Than 80 Years with Cataracts and Corneal Astigmatism. Ophthalmol Ther 12, 1583–1594 (2023). https://doi.org/10.1007/s40123-023-00683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-023-00683-3