Abstract
Background
Currently, there is no generally approved surgical treatment for patients with primary angle-closure glaucoma (PACG) and co-existing cataracts. The aim of this systematic review and meta-analysis was to compare the efficacy and safety of phacoemulsification and phacotrabeculectomy in PACG patients with cataract.
Methods
Diverse databases were searched, including PubMed, MEDLINE, EMBASE, Cochrane Library, Chinese Journal Full-text Database (CNKI), Wanfang database, and China Science and Technology Journal Database, for randomized controlled trials (RCTs) on phacoemulsification and phacotrabeculectomy for the treatment of PACG published up to 30 June 2021. ReviewManager (RevMan) version 5.4 software was used for the meta-analysis, and the effective quantity of measurement data was measured by the mean difference (MD) and 95% confidence interval (CI). The effect of counting data was measured by odds ratio (OR).
Results
Our search of the databases identified 14 RCTs that satisfied the search criteria. Meta-analysis of these 14 RCTs showed that at the 1 month postoperative follow-up, intraocular pressure (IOP) of patients in the phacoemulsification group was higher than that of patients in the phacotrabeculectomy group (MD 2.04, 95% CI 1.42–2.65; P < 0.00001). However, the postoperative IOP was not significantly different between the two groups at the 3, 6, and 12 months postoperative follow-ups (P = 0.52, P = 0.51, and P = 0.05, respectively). More medications for IOP reduction were required by patients in the phacoemulsification group compared with those in the phacotrabeculectomy group at 3 months postoperation (MD 0.76, 95% CI 0.33–1.18; P = 0.0005), 6 months postoperation (MD 0.66, 95% CI 0.15–1.18; P = 0.01), and 12 months postoperation (MD 0.76, 95% CI 0.22–1.30; P = 0.006). Patients in the phacoemulsification group obtained better best corrected visual acuity (BCVA) than those in the phacotrabeculectomy group (MD − 0.17, 95% CI − 0.34 to − 0.01; P = 0.04) at 3 months postoperation, but there was no significant difference in BCVA between the two groups at 6 and 12 months postoperation (P = 0.33 and P = 0.56, respectively). The deepened anterior chamber was more obvious in patients in the phacoemulsification group versus those in the phacotrabeculectomy group (MD 0.61, 95% CI 0.03–1.18; P = 0.04). Patients in the phacoemulsification group experienced fewer postoperative complications than those in the phacotrabeculectomy group (OR 0.27, 95% CI 0.17–0.42; P < 0.00001).
Conclusion
Our results provide evidence that phacotrabeculectomy has advantages over phacoemulsification for the treatment of PACG in terms of better IOP and reduced medication need during the early stage post surgery. However, in terms of the complication risk, phacoemulsification is the more secure treatment option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glaucoma is currently one of the most common causes of blindness worldwide. Trabeculectomy has long been considered the gold standard for controlling intraocular pressure (IOP) in glaucoma patients, but this surgical procedure can result in serious intraoperative and postoperative complications. |
Phacoemulsification is a beneficial surgical procedure for the treatment of primary angle-closure glaucoma (PACG). However, the dilemma facing many ophthalmologists is whether it is necessary to combine lens extraction and filtration surgery in patients with PACG and co-existing cataract. |
We conducted a systematic review and meta-analysis of randomized controlled trials on phacoemulsification and phacotrabeculectomy for the treatment of PACG published up to 30 June 2021, with the aim to compare the efficacy and safety of these two surgical options in patients with PACG and co-existing cataract, and to provide reliable evidence on which to base the choice of operation. |
The results provide evidence that for the treatment of PAGC, phacotrabeculectomy facilitates early IOP control with less use of IOP-lowering agents postoperatively; on the other hand, phacoemulsification is safer and has fewer postoperative complications compared with phacotrabeculectomy. |
Introduction
Glaucoma is one of the leading causes of irreversible blindness worldwide [1]. It has been estimated that 25 million patients currently have primary angle-closure glaucoma (PACG), with the number of cases anticipated to rise to 34 million by 2040 [2]. Therefore, it is particularly important to choose the proper treatment and strategy for managing PACG.
Eyes with PACG frequently present with a number of anatomical risk factors, including small cornea, shallow anterior chamber, short axial length (AL), thick lens, and an anterior lens position [3]. Pupillary block is the main and most commonly known mechanism of anterior chamber angle closing, but it is not the only mechanism. The World Glaucoma Association guidelines point out that the mechanism of angle closure during PACG progression mainly involves the iris, ciliary body, lens, and posterior lens [4]. Angle closure may be caused by multiple mechanisms in some patients with PACG, and a thickened and anteriorly positioned lens plays an important role in the pathogenesis of angle closure in these patients. Several studies have indicated that lens extraction can lower the intraocular pressure (IOP), with high IOP occurring not only in the eyes of individuals with PACG and primary open angle glaucoma (POAG), but also in normal eyes [5,6,7]. Currently, lens extraction has been widely performed clinically and achieved some curative benefits for PACG patients with co-existing cataract. However, there is no consensus on the best surgical approach or on its principles and indications.
Trabeculectomy is the most common procedure in glaucoma surgery for IOP control. However, trabeculectomy has the potential to result in substantial intraoperative and postoperative complications, including low IOP, choroidal detachment, macular lesions and filtering bleb-related complications [8].
Whether to combine lens extraction with trabeculectomy for the treatment of PACG patients with cataract is a controversial and debated issue. In an attempt to clarify this issue, we have performed a systematic search and meta-analysis of pertinent literature and evaluated the efficacy and safety of phacoemulsification and phacotrabeculectomy in the treatment of PACG patients with co-existing cataract. We also provide reliable evidence for selecting a clinical surgical approach.
Methods
Literature Search Strategy
Two of the authors independently searched key databases, including PubMed, Medline, EMBASE, Cochrane Library, CNKI, Wanfang database and China Science, and Technology Journal Database, up to 30 June 2021 for randomized controlled trials (RCTs) on phacoemulsification and phacotrabeculectomy in the treatment of PACG. The following search terms were used: randomized controlled trial; controlled clinical trial; randomized; placebo; randomly; trial; groups; phacotrabeculectomy; trabeculectomy filtering surgery; primary angle closure; angle-closure glaucoma; angle-closure glaucomas; glaucomas, angle-closure; glaucoma, uncompensated; glaucomas, uncompensated; uncompensated glaucoma; uncompensated glaucomas; glaucoma, closed-angle; closed-angle glaucoma; closed-angle glaucomas; glaucoma, closed angle; glaucomas, closed-angle; glaucoma, uncompensative; glaucomas, uncompensative; uncompensative glaucoma; uncompensative glaucomas; glaucoma, angle closure; angle closure glaucoma; angle closure glaucomas; glaucomas, angle closure; glaucoma, narrow-angle; glaucoma, narrow angle; glaucomas, narrow-angle; narrow-angle glaucoma; narrow-gngle glaucomas; glaucoma, angle-closure; cataract extractions; extraction, cataract; extractions, cataract; phakectomy; phakectomies; enzymatic zonulolysis; enzymatic zonulolyses; zonulolyses, enzymatic; zonulolysis, enzymatic; capsulorhexis phacoemulsification; cataract extraction.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Inclusion and Exclusion Criteria
Studies were included in this systematic review and meta-analysis if they satisfied the following criteria: (1) the study examined PACG with co-existing cataract; (2) the study was an RCT; (3) the study compared phacoemulsification with phacotrabeculectomy; (4) the article was published in Chinese or English; (5) the follow-up time was ≥ 3 months. Exclusion criteria were: (1) studies containing interventions other than phacoemulsification or phacotrabeculectomy; (2) studies with incomplete resources, insufficient data, or lack of author information.
Data Extraction
The relevant data from the chosen articles were extracted by two of the authors independently according to the designed data extraction table. Specific information extracted included: (1) general characteristics of the study, including author, publication date, and journal in which the article was published; (2) general characteristics of the subjects included in the study; (3) intervention measures, outcome indicators, among others; and (4) statistical methods used in the research. Inconsistencies and disagreements regarding the data extraction process was resolved by discussion with the third author.
Quality Assessment
The two authors independently assessed the quality of the selected studies according to the pre-determined evaluation criteria. Disagreement was resolved by discussion or by consultation with the third author. The quality of the RCT was evaluated using the Cochrane bias risk tool according to the following seven domains: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (implementation bias); (4) blinding of outcome assessment (measurement bias); (5) incomplete outcome data (follow-up bias); (6) selective reporting of research results (reporting bias); (7) other bias. Each domain was graded into “low risk of bias,” “high risk of bias,” or “unclear risk of bias”.
Statistical Analysis
ReviewManager (RevMan) version 5.4 software was used for all analyses. The heterogeneity among studies was evaluated by the Q test and I2 test. For the studies with significant heterogeneity (P < 0.1 or I2 > 50%), the random-effects model was employed for analysis. When significant heterogeneity existed, sensitivity analysis was utilized to exclude studies with large differences or studies with a high risk of bias in order to test the stability of the combined results. For studies with low heterogeneity (P > 0.1 or I2 < 50%), the fixed-effects model was used for analysis. IOP, the number of agents used to reduce IOP, best correct visual acuity (BCVA), and anterior chamber depth (ACD) were measured. Mean difference (MD) and 95% confidence interval (CI) were used as variables in the statistical analysis. The complications arising during treatment were considered to be binary variables, and the odds ratio (OR) and 95% CI were used in these statistical analyses. A P value < 0.05 was considered to be statistically significant.
Results
Study Selection
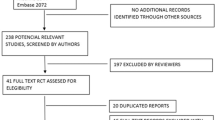
A total of 1587 potentially relevant publications were identified from the database searches, of which 1290 studies remained after duplicates were removed. Of these 1290 studies, 1230 had inconsistent contents or incomplete main indicators and were excluded based on the title and abstract. Sixty full-text articles were identified as potentially relevant for analysis based on the inclusion and exclusion criteria. Ultimately, 14 studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22] involving 992 eyes were included in the analysis. There was no significant difference in age, sex composition ratio, and IOP before treatment between the two treatment groups (phacoemulsification vs. phacotrabeculectomy; P > 0.05). The article screening process is shown in Fig. 1, and basic information on the included studies is shown in Table 1.
Characteristics of the Enrolled Studies
The 14 included studies were all RCTs. The Cochrane Risk of Bias Assessment Tool was applied to evaluate the quality and bias risk of these RCTs, and the results are shown in Fig. 2. Seven studies [11, 15,16,17,18, 20, 21] mentioned the random sequence generation scheme in the text and were therefore judged to have a low risk of bias. Six studies [9, 12,13,14, 19, 22] were assessed as having an unclear risk of bias since they did not specify the random sequence generation scheme in the text. One study [10] was at high risk of bias due to the use of an incorrect random sequence. Seven studies [9, 10, 12,13,14, 19, 22] were at unclear risk of bias because they failed to report the allocation concealment of selection bias. Based on implementation bias, measurement bias, follow-up bias, reporting bias, and other biases, all studies [9,10,11,12,13,14,15,16,17,18,19,20,21,22] were at low risk of bias.
Assessment of the risk of bias in the 14 randomized controlled trials included in the systematic review and meta-analysis. a Judgements on each risk of bias item, presented as percentages across all included studies. b Judgements on each risk of bias item for each included study. A plus sign (+) indicates a low risk of bias, a minus sign (–) indicates a high risk of bias, and a question mark (?) indicates an unclear risk of bias
Meta-analysis Results
IOP at Different Time Points Postoperation
A total of five studies [13, 17, 19,20,21] recorded the IOP values of the two treatment groups 1 month after the operation. Because no statistical heterogeneity was found among these studies (P = 0.40, I2 = 1%), we utilized the fixed-effects model for analysis. The results demonstrated that the IOP in the phacoemulsification group was significantly higher than that in the phacotrabeculectomy group 1 month postoperation and that the difference was statistically significant (MD 2.04, 95% CI 1.42 ~ 2.65; P < 0.00001) (Fig. 3a).
Forest plots comparing the intraocular pressure (IOP) in patients of the phacoemulsification (Phaco) and phacotrabeculectomy groups at different follow-up times. a One month postoperation, b 3 months postoperation, c 6 months postoperation, d 12 months postoperation. CI Confidence interval, SD standard deviation
A total of nine studies [11,12,13,14,15,16,17, 19,20,21] described the IOP values of the two groups of patients at 3 months after treatment. As there was significant heterogeneity between the studies (P < 0.00001, I2 = 93%), the random-effects model was used for analysis. The results showed that there was no significant difference in IOP between the two treatment groups at 3 months postoperation (MD − 0.58, 95% CI − 2.31 to 1.16; P = 0.52 (Fig. 3b).
A total of seven studies [9, 10, 14, 17, 19,20,21] recorded the IOP values of the two groups of patients at 6 months after treatment. There was significant heterogeneity between the studies (P < 0.00001, I2 = 97%), so the random-effects model was employed for the analysis. The results showed that there was no significant difference in IOP between the two treatment groups at 6 months postoperation (MD 1.1, 95% CI − 2.18 to 4.37; P = 0.51) (Fig. 3c).
A total of five studies [17, 19,20,21] reported the IOP values of the two groups of patients at 12 months after treatment. There was significant heterogeneity between the studies (P = 0.04, I2 = 59%), so the random-effects model was used for analysis. The results showed that there was no significant difference in IOP between the two treatment groups at 12 months postoperation (MD 1.21, 95% CI 0.02–2.40; P = 0.05 (Fig. 3d).
Agents for IOP Reduction at Different Time Points Postoperation
A total of four studies [17, 18, 20, 21] recorded the usage of agents for reducing IOP in the two groups of patients at 3 months postoperation. As there was significant heterogeneity between the studies (P = 0.04, I2 = 69%), the random-effects model was applied for analysis. The results showed that at 3 months postoperation, patients in the phacoemulsification group required more agents for IOP reduction than patients in the phacotrabeculectomy group, and that the difference was statistically significant (MD 0.76, 95% CI 0.33–1.18; P = 0.0005) (Fig. 4a).
A total of four studies [17, 18, 20, 21] reported the usage of agents for IOP reduction in the two groups of patients at 6 months postoperation. There was significant heterogeneity between the studies (P = 0.008, I2 = 79%), so the random-effects model was applied for analysis. The results showed that at 6 months postoperation, patients in the phacoemulsification group required more agents for IOP reduction than patients in the phacotrabeculectomy group, and that the difference was statistically significant (MD 0.66, 95% CI 0.15–1.18; P = 0.01) (Fig. 4b).
A total of five studies [17, 18, 20,21,22] recorded the usage of agents for IOP reduction in the two groups of patients at 12 months postoperation. As there was significant heterogeneity between the studies (P = 0.0004, I2 = 83%), the random-effect models was applied for analysis. The results showed that at 12 months postoperation, patients in the phacoemulsification group required more agents for IOP reduction than patients in the phacotrabeculectomy group, and that the difference was statistically significant (MD 0.76, 95% CI 0.22–1.30; P = 0.006) (Fig. 4c).
BCVA postoperation
A total of three studies [11, 12, 15] recorded the BCVA of the two groups at 3 months postoperation. There was no significant heterogeneity between the studies (P = 0.23, I2 = 33%), so the fixed-effects model was applied for analysis. The results showed that the patients in the phacoemulsification group obtained better BCVA than those in the phacotrabeculectomy group, and that the difference was statistically significant (MD − 0.17, 95% CI − 0.34 to − 0.01; P = 0.04 (Fig. 5a).
A total of two studies [10, 19] recorded the BCVA of the two groups at 6 months postoperation. There was significant heterogeneity between the studies (P = 0.001, I2 = 90%), so the random-effects model was applied for analysis. The results revealed that there was no significant difference in the BCVA at 6 months postoperation between the two groups (MD 0.18, 95% CI − 0.19 to 0.56; P = 0.33) (Fig. 5b).
A total of five studies [17, 19,20,21,22] recorded the BCVA of the two groups at 12 months postoperation. As there was no significant heterogeneity between the studies (P = 0.53, I2 = 0%), the fixed-effects model was applied for analysis. The results showed that there was no significant difference in the BCVA at 12 months postoperation between the two groups (MD = 0.03, 95% CI − 0.13 to 0.07; P = 0.56) (Fig. 5c).
ACD Postoperation
A total of five studies [9, 10, 13, 16, 19] recorded the ACD of the two treatment groups postoperation. There was significant heterogeneity between the studies (P < 0.00001, I2 = 99%), so the random-effects model was applied for analysis. The results showed that the central ACD of patients in the phacoemulsification group was significantly deeper than that of patients in the phacotrabeculectomy group at 3 months postoperation, and that the difference was statistically significant (MD 0.61, 95% CI 0.03–1.18; P = 0.04) (Fig. 6).
Probability of Complications During Treatment
A total of nine studies [9, 11, 12, 14,15,16,17, 20, 21] recorded the complications that arose during postoperative treatment; these included corneal edema, hyphema, shallow anterior chamber, increased IOP, and choroidal detachment. There was no significant heterogeneity between the studies (P = 0.16, I2 = 32%), so the fixed-effects model was applied for analysis. The results showed that the incidence of complications in patients of the phacoemulsification group was significantly lower than that in the phacotrabeculectomy group, and that the difference was statistically significant (OR 0.27, 95% CI 0.17–0.42; P < 0.00001) (Fig. 7).
Discussion
The development of phacoemulsification as a cataract surgery method has been highly beneficial in the treatment of glaucoma. Studies have revealed that: (1) implantation of an intraocular lens with a thickness of < 1.0 mm instead of an opaque lens with a thickness of 4.5–5.5 mm can significantly deepen the depth of the anterior chamber, re-open the anterior chamber angle, release the pupil block, and improve vision [23]; (2) intraoperative high perfusion pressure and viscoelastic substances can flush and open the anterior chamber angle or reduce adhesions [24]; (3) ultrasound oscillation and high perfusion pressure can promote the dissolution of glycosaminoglycan in the trabecular meshwork, enhance the phagocytic function of trabecular cells, increase the permeability of the trabecular meshwork, and ultrasound can also reduce the secretion of the ciliary body and the production of aqueous humor [25]; and (4) the increased level of inflammatory mediators in the aqueous humor after phacoemulsification can contribute to the opening of the trabecular meshwork pathway and uveoscleral outflow pathway to enhance the aqueous humor drainage [26]. Phacoemulsification has become a modern and important approach for glaucoma treatment by effectively treating the pathogenesis of PACG, obtaining better visual function improvement, reconstructing the anterior chamber angle, and improving the outflow facility [27,28,29]. In recent years, an increasing number of ophthalmologists have realized that lens surgery can be applied in some cases that were previously thought to require filtration surgery.
Regardless of the surgical methods, lowering IOP remains the main goal of treatment for glaucoma patients. Although lens extraction can significantly lower the IOP [30,31,32], published studies have demonstrated that cataract extraction alone results in less IOP reduction than cataract surgery combined with trabeculectomy [20].
The important question which remains for ophthalmologists treating patients with PACG and cataracts is whether lens extraction combined with filtering surgery necessary. A number of clinical studies have shown that phacotrabeculectomy provides no advantage over lens extraction alone in terms of regulating IOP and minimizing the number of agents for IOP reduction [33, 34]. In these same studies, the phacotrabeculectomy group experienced more complications, such as low IOP, than the phacoemulsification group. As early as 2013, a systematic evaluation of PACG combined with cataracts pointed out that postoperative vision in the phacoemulsification group was better than that in the phacotrabeculectomy group [35], and that the difference was statistically significant. However, there was no significant difference in postoperative IOP between the two groups. It has demonstrated that phacoemulsification can be utilized to treat patients with PACG and cataracts.
In our study, we found that the IOP in the phacoemulsification group at 1 month postoperation was higher than that in the phacotrabeculectomy group at the same time point (MD 2.04, 95% CI 1.42–2.65; P < 0.00001). However, there was no significant difference in IOP between the two groups at 3 months postoperation (MD − 0.58, 95% CI − 2.31 to 1.16; P = 0.52), 6 months postoperation (MD 1.1, 95% CI − 2.18 to 4.37; P = 0.51), and 12 months postoperation (MD 1.21, 95% CI 0.02–2.40; P = 0.05). This result implied that phacotrabeculectomy had a significant effect on lowering IOP during the early postoperative stage but that there was no significant difference in IOP between the two groups at later stages of the follow-up. Previous studies showed that phacotrabeculectomy had no advantage over phacoemulsification in terms of postoperative IOP control [33,34,35]; this result is not completely consistent with the results of our study. This difference may be due to the different follow-up times selected for evaluation. In this systematic review and meta-analysis, we selected the time points of 1, 3, 6, and 12 months postoperation to evaluate the IOP of the two groups, while previous studies only evaluated the postoperative IOP generally. Our results also showed that patients in the phacoemulsification group required more agents for IOP reduction than did patients in the phacotrabeculectomy group at 3 months postoperation (MD 0.76, 95% CI 0.33–1.18; P = 0.0005), 6 months postoperation (MD 0.66, 95% CI 0.15–1.18; P = 0.01), and 12 months postoperation (MD 0.76, 95% CI 0.22–1.30; P = 0.006). These results showed that phacotrabeculectomy had more advantages than phacoemulsification in minimizing the use of agents for IOP reduction; these results are inconsistent with those of previous systematic evaluations [33, 34]. With the advancement of medical technology, the variety of agents available for IOP reduction has increased in recent years, providing more options for PACG patients whose postoperative IOP is not well controlled. However, in the more distant past, patients with poor postoperative IOP control may have required a second operation, which could explain why the results of the present study contradict those of previous systematic evaluations. In the present study, the patients in the phacoemulsification group obtained better BCVA than those in the phacotrabeculectomy group (MD 0.61, 95% CI 0.03–1.18; P = 0.04); however, there was no significant difference in the BCVA between the groups at 6 months postoperation (MD 0.18, 95% CI − 0.19 to 0.56; P = 0.33) and 12 months postoperation (MD 0.03, 95% CI − 0.13 to 0.07; P = 0.56). Due to the small surgical trauma experienced by the patient undergoing phacoemulsification, as well as the quick postoperative recovery, the vision of patients in the phacoemulsification group was better than that of patients in the phacotrabeculectomy group during the early follow-up period; there was no significant difference in BCVA between the two groups at the later follow-ups. Three-month postoperation, the central ACD in the phacoemulsification group was significantly deeper than that in the phacotrabeculectomy group (MD 0.61, 95% CI 0.03–1.18; P = 0.04). Deepened ACD can effectively reduce the formation of peripheral angle adhesion (PAS) and improve IOP control. The incidence of complications was significantly lower in patients of the phacoemulsification group compared to those in the phacotrabeculectomy group (OR 0.27, 95% CI 0.17–0.42' P < 0.00001). The main complications were corneal edema, hyphema, shallow anterior chamber, increased IOP, and choroidal detachment, which is consistent with the results of previous systematic reviews [33, 34].
Based on the evidence presented here, phacoemulsification may be an important surgical option for the treatment of PACG. Compared with phacotrabeculectomy, phacoemulsification has less advantage in IOP control, but the phacoemulsification group had significantly fewer postoperative complications. For PACG patients with cataracts, there are benefits and drawbacks to both phacotrabeculectomy and phacoemulsification, so multiple factors should be considered when determining the most appropriate surgery. Phacoemulsification may be preferred if patients cannot accept the risk of filtering surgery complications or have high risk factors, such as corneal endothelial dysfunction. On the other hand, if the patients are known to have poor medication compliance or tolerance, or if long-term medication or follow-up is difficult to achieve, phacotrabeculectomy may be more beneficial. There are currently few articles evaluating the long-term efficacy of the two treatments. Therefore, it is urgent to conduct international or domestic standard, large-scale, multicenter, randomized clinical control studies to assess the safety and efficacy of these two treatments.
There are a number of limitations to this study. First, the sample size of the selected study was small. Secondly, since the number of selected studies of each observation index was < 10, we did not use a funnel chart to detect publication bias. Third, the changes in the visual field and the degree of PAS at different postoperative times were the indicators used to evaluate the two treatments. Because we did not obtain sufficient data, we did not analyze these factors in the present study. In the future, more large-scale clinical randomized controlled studies with long-term follow-ups are needed to analyze the efficacy of the two treatments of PACG.
Conclusions
This meta-analysis provides sufficient evidence that phacotrabeculectomy has advantages over phacoemulsification in the treatment of PACG in terms of IOP and medication reduction in the early postoperative stage. However, phacoemulsification is the more secure method in terms of reducing complications.
References
World Health Organization. Global data on visual impairments. 2010. www.who.int/blindness/GLOBALDATAFINALforweb.pdf.
Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Sun X, Dai Y, Chen Y, et al. Primary angle closure glaucoma: What we know and what we don’t know. Prog Retin Eye Res. 2017;57:26–45.
Ningli W, Renyi W, Huiping Y, et al. WGA consensus series. Chin Med J. 2016;51(11):113.
Baek SU, Kwon S, Park IW, et al. Effect of phacoemulsification on intraocular pressure in healthy subjects and glaucoma patients. J Korean Med Sci. 2019;34(6): e47.
Shingleton BJ, Gamell LS, O’Donoghue MW, et al. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–90.
Ngo WK, Tan CS. Effect of bilateral sequential cataract extraction on intraocular pressure in non-glaucomatous Asian eyes. Br J Ophthalmol. 2016;100(4):560–4.
Salmon JF. The role of trabeculectomy in the treatment of advanced chronic angle-closure glaucoma. J Glaucoma. 1993;2(4):285–90.
Xiaoxia W, Yanhua Z. Clinical observation of phacoemulsification combined with intraocular lens implantation in the treatment of primary angle closure glaucoma complicated with senile cataract. J Tradit Chin Med Sci. 2014;42(5):40–2.
Shaona C, Junwei L, Xiaoquan C, et al. Comparison of effectiveness of phacoemulsification and combined glaucoma-cataract surgery in closed angle glaucoma with cataract. China Med Eng. 2020;28(9):73–6.
Xianfeng L, Hui C, Juanhui C, et al. Comparison of different surgical methods in the treatment of acute angle-closure glaucoma and cataract. Contemp Med. 2019;25(23):83–5.
Guifen G. Effect of phacoemulsification on primary angle closure glaucoma patients with cataract. Contemp Med Forum. 2019;17(24):15–6.
Junyi X, Shigang Y, Haoquan L, et al. The clinical analysis of phacoemulsification combined with intraocular lens implantation in treating cataract and angle-closure glaucoma. China Med Pharmacy. 2014;4(10):190–2.
Xinxing Z. Comparison of different surgical methods in the treatment of primary angle closure glaucoma complicated with cataract. Guide China Med. 2016;14(36):137–8.
Yuqiang G, Qingsheng Z, Yanli P, et al. Efficacy evaluation of phacoemulsification with IOL implantation in patients with acute angle-closure glaucoma complicated with cataract. Int Eye Sci. 2018;18(12):2238–40.
Meixia L. Evaluation of the curative effect of simple cataract surgery in the treatment of angle-closure glaucoma combined with cataract. Syst Med. 2021;6(11):42–4.
El Sayed YM, Elhusseiny AM, Albalkini AS, et al. Mitomycin C-augmented phacotrabeculectomy versus phacoemulsification in primary angle-closure glaucoma: a randomized controlled study. J Glaucoma. 2019;28(10):911–5.
Chelerkar V, Parekh P, Kalyani VKS, et al. Comparative clinical study of medically controlled nonsevere chronic primary angle-closure glaucoma with coexisting cataract surgically managed by phacoemulsification as against combined phacotrabeculectomy. Middle East Afr J Ophthalmol. 2018;25(3–4):119–25.
Hou X, Hu D, Cui Z, et al. Small-incision phacotrabeculectomy versus phacoemulsification in refractory acute primary angle closure with cataract. BMC Ophthalmol. 2015;15:88.
Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology. 2009;116(4):725–31.
Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology. 2008;115(12):2167–73.
Tham CC, Leung DY, Kwong YY, et al. Effects of phacoemulsification versus combined phaco-trabeculectomy on drainage angle status in primary angle closure glaucoma (PACG). J Glaucoma. 2010;19(2):119–23.
Xudong S, Ningli W, Guangxian T, et al. Multi-centre trial of phacoemulsification on patients with primary angle-closure glaucoma and co-existing cataract. J Med Res. 2010;39(3):17–22.
Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma ( PACG) and co-existing cataract: a prospective case series. J Glaucoma. 2006;15(1):47–52.
Hu XK, Weng JN, Zhuang P. Study of surgical treatment of cataract related to angle-closure glaucoma. Guoji Yanke Zazhi (Int Eye Sci). 2012;12(3):458–60.
Wang N, Chintala SK, Fini ME, et al. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44(5):1977–81.
Zhang X, Teng L, Li A, et al. The clinical outcomes of three surgical managements on primary angle-closure glaucoma. Yan Ke Xue Bao. 2007;23(2):65–74.
Ge J, Zhang X, Giaconi JA, et al. Angle-closure glaucoma: surgical management of acute angle-closure glaucoma. Pearls of Glaucoma Management. Berlin: Springer; 2010. p. 439–44.
Ke Y, Wei W, Wei W, et al. Clinical evaluation on the coaxial 1.8 mm microincision cataract surgery. Chin J Ophthalmol. 2011;47(10):903–7.
Harasymowycz PJ, Papamatheakis DG, Ahmed I, et al. Phacoemulsification and goniosynechialysis in the management of unresponsive primary angle closure. J Glaucoma. 2005;14(3):186–9.
Matsumura M, Ido W, Shirakam Y, Zoizumi K. Treatment of primary closed angle glaucoma with cataract by lysis of peripheral anterior synechiae and intraocular lens implantation. Jpn J Clin Ophthalmol. 1991;45:1567–9.
Teekhasaenee C, Ritch R. Combined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucoma. Ophthalmology. 1999;106(4):669–74.
Deng BL, Jiang C, Ma B, et al. Surgical treatment for primary angle-closure glaucoma: a meta analysis. Int J Ophthalmol. 2011;4(3):223–7.
Wang F, Wu ZH. Phacoemulsification versus combined phacotrabeculectomy in the treatment of primary angle closure glaucoma with cataract: a meta-analysis. Int J Ophthalmol. 2016;9(4):597–603.
Shaodan S, Jing L, Wei Z, et al. Systematic review of surgical treatment for primary angle-closure glaucoma accompanied with Cataract. J Otolaryngol Ophthal Shandong Univ. 2013;27(5):84–90.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The authors are funding the journal’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Bo Han. Methodology: Bo Han and Jia Xie. Formal analysis and investigation: Jia Xie and Wan Li. Writing—original draft preparation: Bo Han and Jia Xie. Writing—review and editing: Bo Han, Jia Xie, and Wan Li. Resources: Bo Han. Supervision: Bo Han.
Disclosures
Jia Xie, Wan Li, and Bo Han declare that they have no competing interests.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xie, J., Li, W. & Han, B. The Treatment of Primary Angle-Closure Glaucoma with Cataract: A Systematic Review and Meta-Analysis of Randomized Controlled Trails. Ophthalmol Ther 12, 675–689 (2023). https://doi.org/10.1007/s40123-022-00639-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00639-z